Chitosan nanoparticle preparation of ceftiofur sodium, and preparation method thereof
A technology of chitosan nanoparticles and ceftiofur sodium, which is applied in the directions of antibacterial drugs and powder transportation, can solve the problems of poor physical stability, easy sedimentation, short maintenance time, etc., and achieves simple and reliable preparation method and low price. Inexpensive, quality controllable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Dissolve 3 mg of chitosan in 10 ml of 0.3% acetic acid solution to obtain a chitosan solution (solution A); dissolve 4.52 mg of sodium tripolyphosphate and 10 mg of ceftiofur sodium in 4 ml of deionized water to obtain a solution containing The sodium tripolyphosphate solution (solution B) of ceftiofur sodium, under the condition of magnetic stirring, solution B is slowly added dropwise in the solution A, continue stirring for 30min to obtain the ceftiofur sodium chitosan nanoparticle preparation, and the stirring rate is 400rpm.
example 2
[0023] Dissolve 7 mg of chitosan in 10 ml of 0.3% acetic acid solution to obtain a chitosan solution (solution A); dissolve 10.52 mg of sodium tripolyphosphate and 20 mg of ceftiofur sodium in 4 ml of deionized water to obtain a solution containing The sodium tripolyphosphate solution (solution B) of ceftiofur sodium, under the condition of magnetic stirring, solution B is slowly added dropwise in the solution A, continue stirring for 60min to obtain the ceftiofur sodium chitosan nanoparticle preparation, and the stirring rate is 1200rpm.
example 3
[0025] Dissolve 4 mg of chitosan in 10 ml of 0.3% acetic acid solution to obtain a chitosan solution (solution A); dissolve 6 mg of sodium tripolyphosphate and 8 mg of ceftiofur sodium in 4 ml of deionized water to obtain a solution containing cephalosporin Sodium tripolyphosphate solution (solution B) of sodium thiofur, under magnetic stirring conditions, slowly add solution B dropwise to solution A, continue stirring for 10 minutes to obtain ceftiofur sodium chitosan nanoparticle preparation, stirring speed is 800rpm .
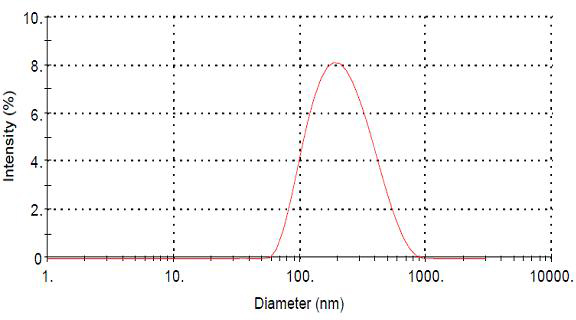
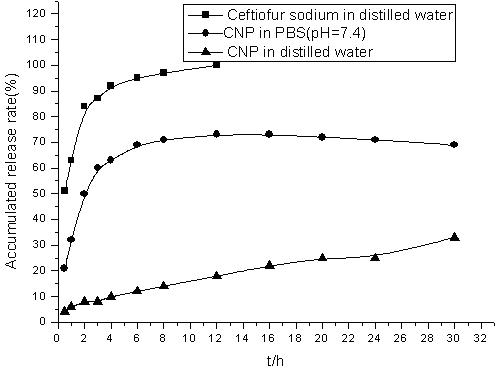
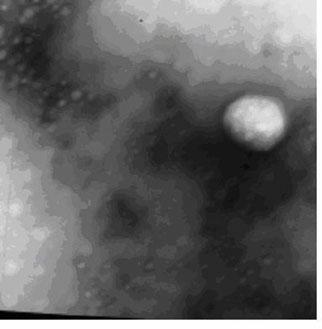
[0026] Characterization of ceftiofur sodium chitosan nanoparticle preparation:
[0027] Observation of appearance
[0028] Take the colloidal solution of ceftiofur sodium chitosan nanoparticle preparation, add an appropriate amount of ultrapure water (0.22 μm filter) to dilute, negatively stain with 1.5% (w / v) phosphotungstic acid, drop it on the coated electron microscope copper grid, After drying, observe the appearance of nanoparticles under a transmiss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com