Ceftiofur hydrochloride powder injection as well as preparation method and application thereof
A technology of ceftiofur hydrochloride and ceftiofur, which is used in powder delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc. problems, to achieve the effect of no incompatibility, prolonged concentration action time, and increased feed intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
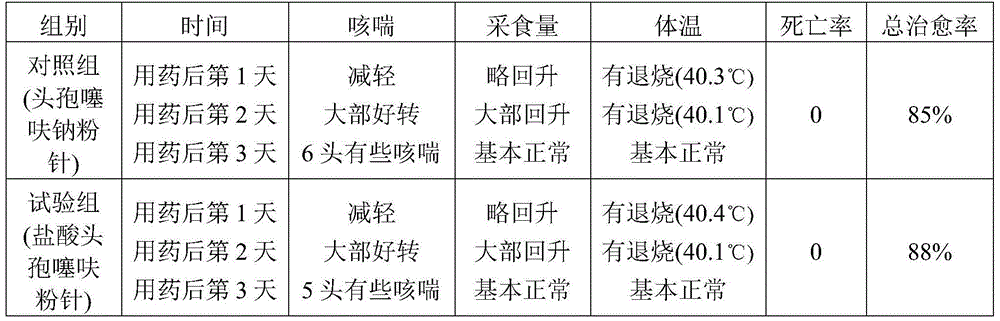
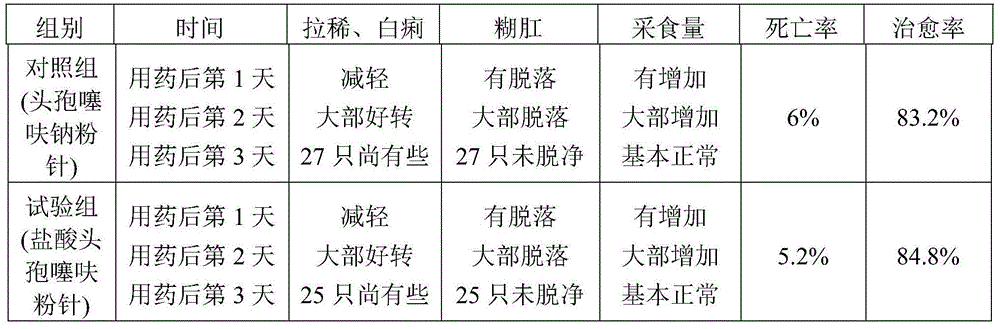
Examples
Embodiment 1
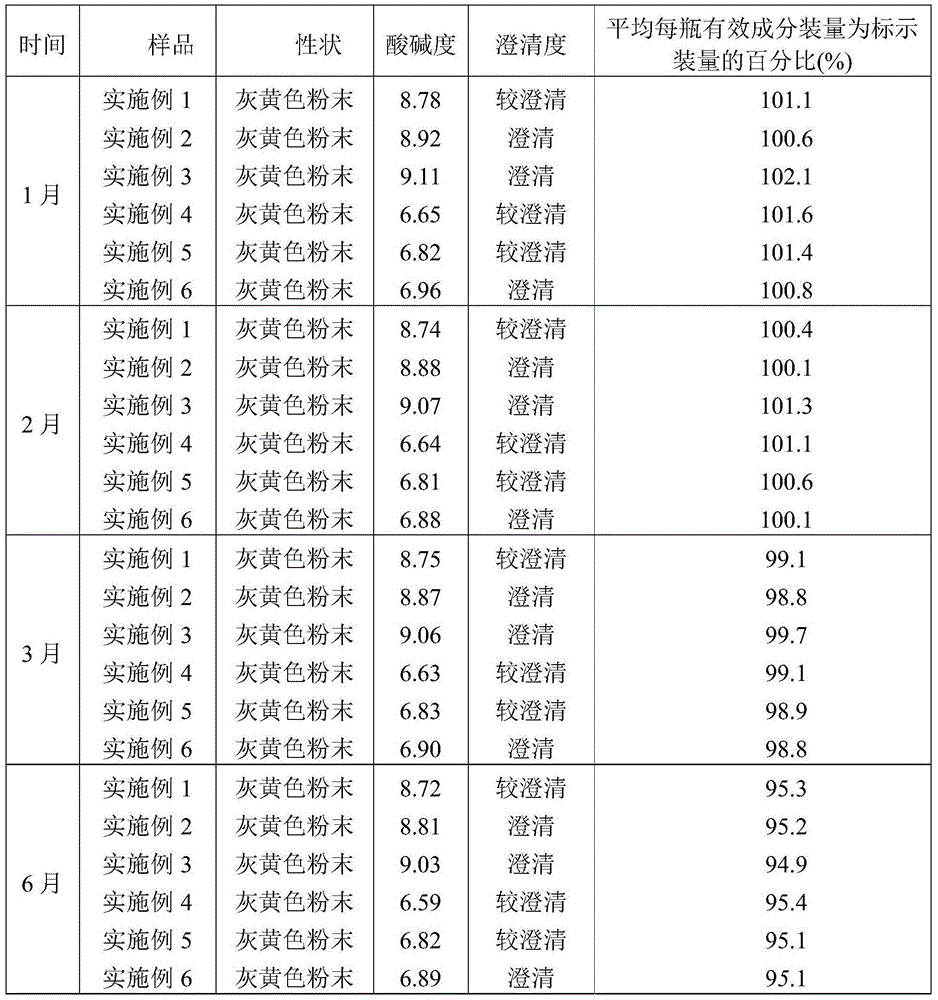
[0027] A preparation method of ceftiofur hydrochloride powder injection, comprising the following steps: weighing ceftiofur hydrochloride and sodium carbonate at a weight ratio of 48 (calculated as the active ingredient ceftiofur):28, and putting it into SYH-100 three-dimensional motion In the mixer, after mixing for 15 minutes, transfer to the KGL-120 type antibiotic glass bottle screw dispensing machine for subpackaging to obtain ceftiofur hydrochloride powder injection. The volume of each bottle is 0.1g, 0.2g, 0.5g or 1.0g (both based on the active ingredient ceftiofur), of which, 0.1g and 0.2g specifications are packed in 5-7mL vials, and 0.5g specifications are used in 10-15mL Divided into vials, 1.0g in 20-25mL vials.
[0028] The particle size of ceftiofur hydrochloride is 60-100 mesh.
[0029] The particle size of sodium carbonate is 60-100 mesh.
[0030] The above operations are all carried out in the powder injection purification workshop, and the cleanliness requi...
Embodiment 2
[0032] A preparation method of ceftiofur hydrochloride powder injection, comprising the following steps: weighing ceftiofur hydrochloride and sodium carbonate at a weight ratio of 50:31 (calculated as the active ingredient ceftiofur), and putting them into a SYH-100 three-dimensional motion In the mixer, after mixing for 15 minutes, transfer to the KGL-120 type antibiotic glass bottle screw dispensing machine for subpackaging to obtain ceftiofur hydrochloride powder injection. The volume of each bottle is 0.1g, 0.2g, 0.5g or 1.0g (both based on the active ingredient ceftiofur), of which, 0.1g and 0.2g specifications are packed in 5-7mL vials, and 0.5g specifications are used in 10-15mL Divided into vials, 1.0g in 20-25mL vials.
[0033] The particle size of ceftiofur hydrochloride is 60-100 mesh.
[0034] The particle size of sodium carbonate is 60-100 mesh.
[0035] The above operations are all carried out in the powder injection purification workshop, and the cleanliness r...
Embodiment 3
[0037] A preparation method of ceftiofur hydrochloride powder injection, comprising the following steps: weighing ceftiofur hydrochloride and sodium carbonate at a weight ratio of 52:34 (calculated as the active ingredient ceftiofur), and putting them into a SYH-100 three-dimensional motion In the mixer, after mixing for 15 minutes, transfer to the KGL-120 type antibiotic glass bottle screw dispensing machine for subpackaging to obtain ceftiofur hydrochloride powder injection. The volume of each bottle is 0.1g, 0.2g, 0.5g or 1.0g (both based on the active ingredient ceftiofur), of which, 0.1g and 0.2g specifications are packed in 5-7mL vials, and 0.5g specifications are used in 10-15mL Divided into vials, 1.0g in 20-25mL vials.
[0038] The particle size of ceftiofur hydrochloride is 60-100 mesh.
[0039] The particle size of sodium carbonate is 60-100 mesh.
[0040] The above operations are all carried out in the powder injection purification workshop, and the cleanliness r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com