Flavone glycoside derivatives, and preparation method and application thereof
A technology of flavonoid glycosides and flavone glycosides, which is applied in the field of pharmacy and can solve problems such as organ damage and toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
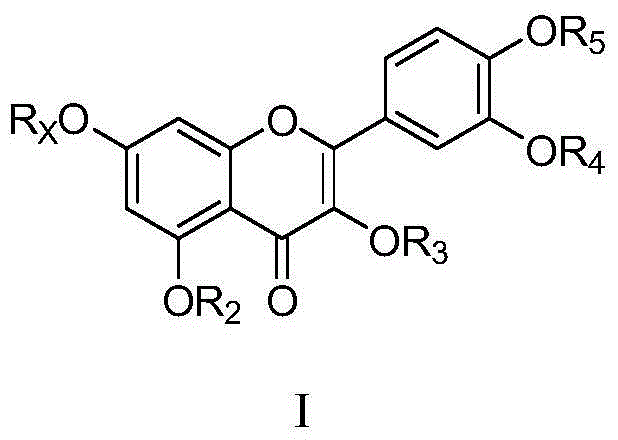
[0030] Example 1 Synthesis of aglycone intermediate 7,4'-di-hydroxyl-3,5,3'-tri-methoxy-flavone (M-04)
[0031]
[0032] Reagents and conditions: (i) BnBr, K 2 CO 3 , DMF, room temperature; (ii) concentrated hydrochloric acid, ethanol, 70 ° C, 3h. (iii) CH 3 I, K 2 CO 3 , DMF, room temperature, 8h; (iv) H 2 , 10% Pd / C, THF, room temperature 15h.
[0033] Preparation steps: (i) Weigh the obtained anhydrous rutin (50g, 80mmol) into a 1000mL reaction bottle, add 600mL anhydrous DMF, stir and dissolve, then add anhydrous K 2 CO 3 (23.8g, 168mmol, 2.1eq) and benzyl bromide (19.4mL, 160mmol, 2eq). React overnight at room temperature, pour the resulting reaction solution into an ice-water bath, add HCl to adjust the pH to 6, a large amount of precipitate precipitates, filter, and wash the filter cake with water to obtain M-01; (ii) take the crude M-01 obtained in the previous step and use Heat and dissolve 600mL of ethanol in water, then add 200mL of concentrated hydrochl...
Embodiment 2
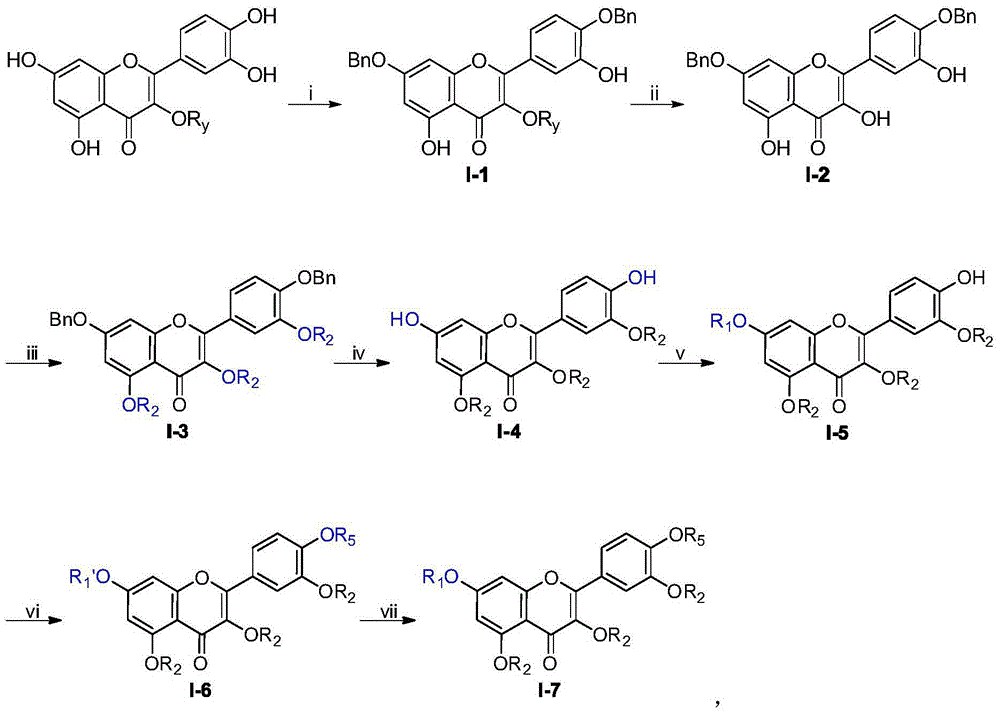
[0034] Example 2 Synthesis of aglycon intermediate 7,4'-di-hydroxy-3,5,3'-tri-ethoxy-flavone (M-06)
[0035]
[0036] Reagents and conditions: (i) CH 3 CH 2 I, K 2 CO 3 , DMF, room temperature, 8h; (ii) H 2 , 10% Pd / C, THF, room temperature 15h.
[0037] Preparation steps: (i) Weigh the aforementioned synthetic intermediate M-02 (10g, 20.7mmol) into a 500mL reaction bottle, add 150mL of anhydrous DMF to dissolve completely, and then add anhydrous K 2 CO 3 (25.8g, 186mmol, 9eq) and CH 3 CH 2 I (6.6mL, 82.8mmol, 4eq), after reacting at 40°C for 8h, the reaction solution was dispersed with ethyl acetate and water, extracted, and the obtained organic layer was washed successively with dilute hydrochloric acid (0.1M), water and saturated brine. Dry over magnesium sulfate and concentrate, and the residue is purified by silica gel column chromatography to obtain the pure product M-05 in the form of light yellow powder with a yield of 90%. (ii) Weigh 10g (17.7mmol) of M-05...
Embodiment 3
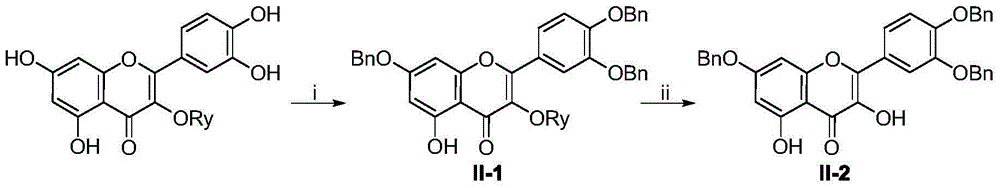
[0038] Example 3 Synthesis of aglycone intermediate 3,5-di-hydroxyl-7,3',4'-tri-benzyloxy-flavone (M-08)
[0039]
[0040] Reagents and conditions: (i) BnBr, K 2 CO 3 , DMF, room temperature; (ii) ethanol, concentrated hydrochloric acid, 70 ° C, 3h.
[0041] Preparation steps: (i) Weigh the obtained anhydrous rutin (50g, 0.08mol) into a 1000mL reaction bottle, add 600mL anhydrous DMF, stir to dissolve, then add anhydrous K 2 CO 3 (56g, 0.41mol, 5eq) and benzyl bromide (36.9mL, 0.3mol, 3.8eq), react at room temperature for 2.5 days, after thin layer chromatography (TLC) detects that the reaction is complete, pour the reaction solution into an ice-water bath and wash with concentrated hydrochloric acid Adjust the pH to 6, precipitate a large amount of precipitate, filter, and the filter cake is the intermediate M-07 crude product; (ii) directly dissolve the M-07 crude product with absolute ethanol (600mL) and add concentrated hydrochloric acid (200mL) to react The liquid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com