Preparation method of amygdalin
The technology of amygdalin and amygdalin is applied in the field of preparation of amygdalin, which can solve the problems of restricting the in-depth research of amygdalin medicines, not preparing D-amygdalin or L-amygdalin epimers separately, and the like. , to achieve the effect of low cost, environmental friendliness and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Extraction: add 20 grams of bitter almonds (commercially available) to 5 times the amount of water, boil for 1 hour, repeat 2 times, combine the extracts, and concentrate under reduced pressure to obtain the crude extract of amygdalin.
[0035] 2. Extraction, extract the crude extract obtained in step 1 with ethyl acetate at a ratio of 1:1 by volume, repeat twice, and then extract three times with n-butanol at a ratio of 1:1 by volume. The n-butanol extract was collected and concentrated under reduced pressure to obtain the amygdalin extract.
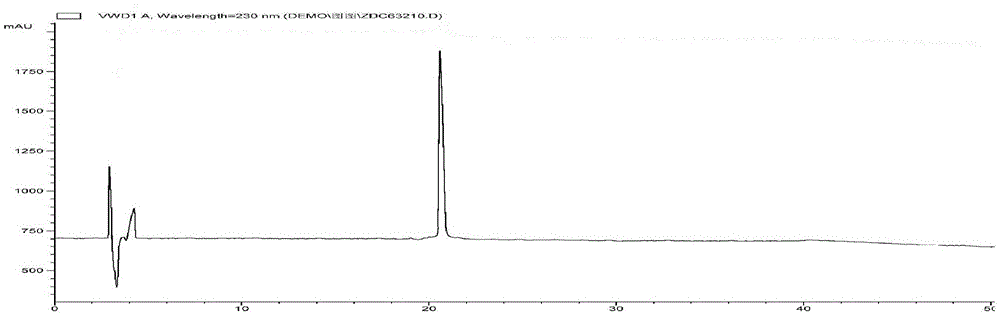
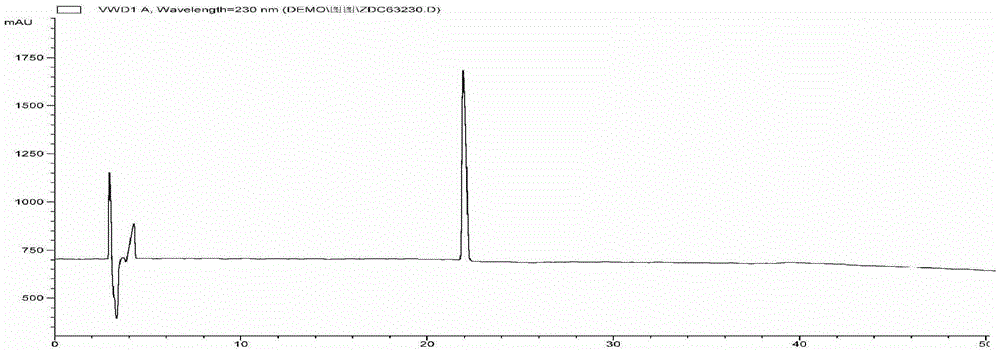
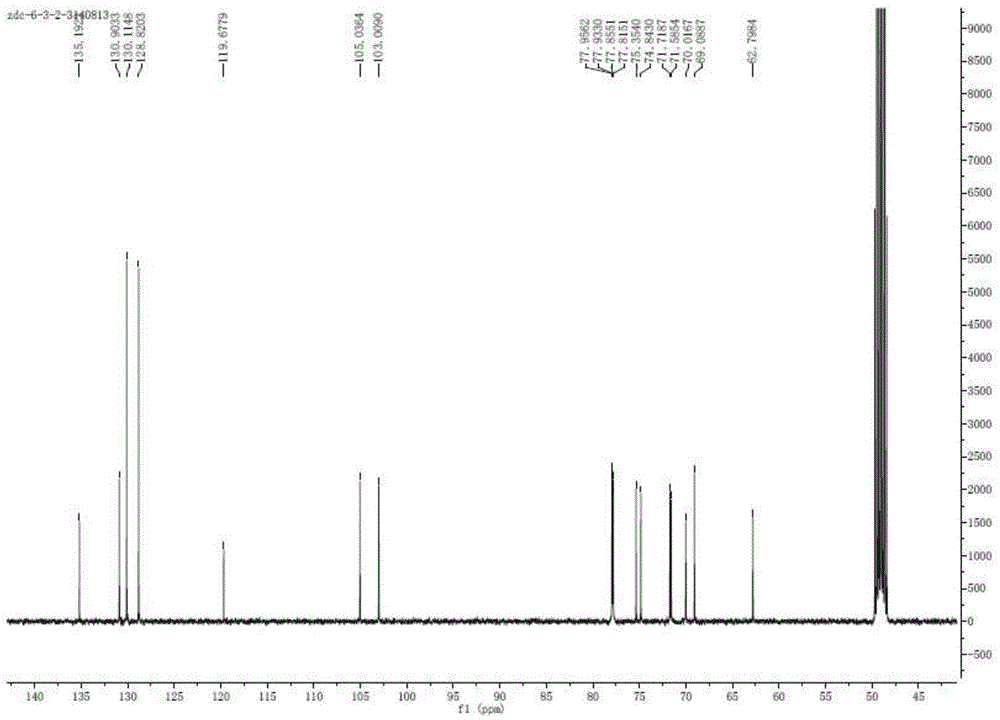
[0036] 3. Dynamic axial compression reverse chromatographic separation (DAC-HB80 Jiangsu Hanbang Technology Co., Ltd.). The above extract was separated by compression chromatography, using C 18 Chromatographic column (250mmx50mm, 10μm), eluting with 15% acetonitrile-0.5% formic acid water as the mobile phase by mass percentage, flow rate: 30ml / min, column temperature: room temperature, pH value: 2.0, detection wavelength: 210...
Embodiment 2
[0049] 1. Extraction. Add 2 kg of peach kernels (commercially available) to 8 times the amount of water, boil for 2 hours, repeat 3 times, combine the extracts, and concentrate under reduced pressure to obtain the crude extract of amygdalin.
[0050] 2. Extraction, the crude extract obtained in step 1 was extracted with ethyl acetate at a volume ratio of 1:1, repeated 3 times, and then extracted with n-butanol at a volume ratio of 1:1 for 3 times. Take n-butanol extract, concentrate under reduced pressure to obtain amygdalin extract.
[0051] 3. Dynamic axial compression reverse chromatographic separation (DAC-HB80 Jiangsu Hanbang Technology Co., Ltd.). The above extract was separated by compression chromatography, using C 18 Chromatographic column (250mmx50mm, 10μm), eluted with 11% acetonitrile-0.2% formic acid water as mobile phase, flow rate: 100ml / min, column temperature: room temperature, pH value: 5.0, detection wavelength: 210nm, injection volume: 5000μL . Observe t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com