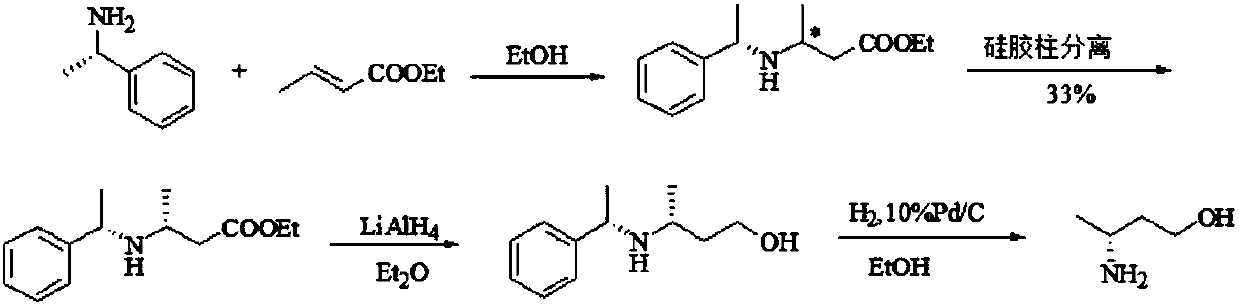
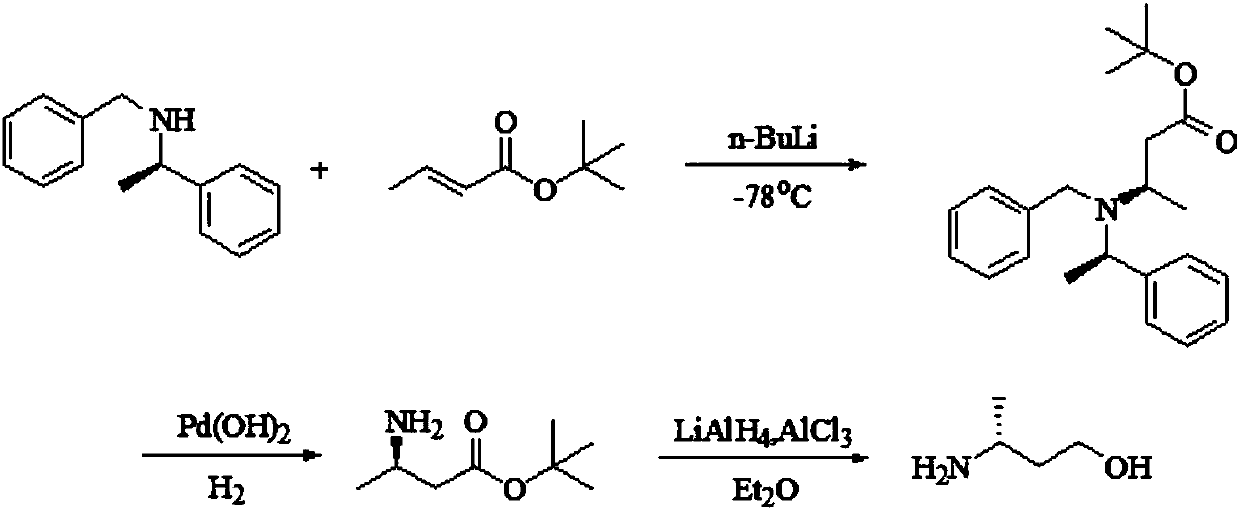
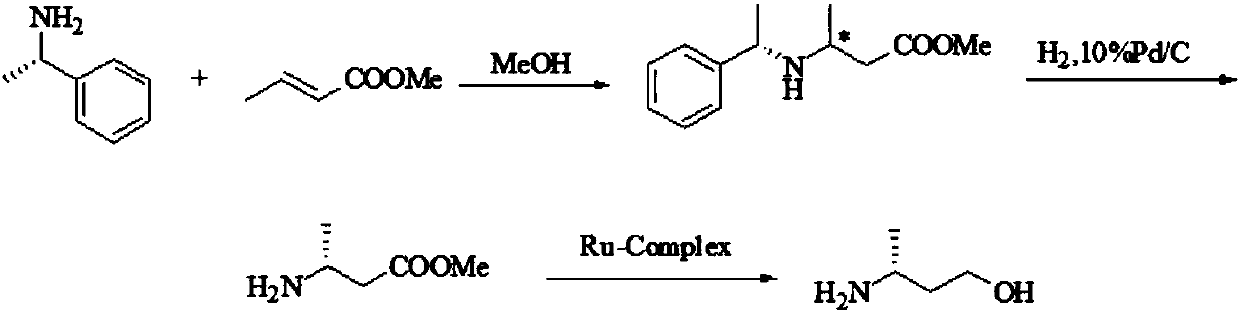
Preparation method of (R)-3-amino butanol
A technology of aminobutanol and butanol, which is applied in the preparation of aminohydroxy compounds, the preparation of organic compounds, organic chemical methods, etc., can solve the problems of waste of raw materials in industrialized large-scale production, toxicity of raw materials, low yield, etc., and achieve environmental protection , short process route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] 1) Amination reduction reaction: In a reaction kettle, add 121g (R)-1-methylbenzylamine, 1184g ethanol, 105.6g butanol and 12.1g Pd / C catalyst (5%). After sequentially replacing with nitrogen and hydrogen, react at a pressure of 4 MPa and a temperature of 50° C. for 12 hours. GC analysis of the starting material is complete. After the reaction was completed, the Pd / C catalyst was filtered, and the filter cake was rinsed with 160 g of ethanol, and then vacuum-dried at 40° C. to obtain 12.2 g of recovered Pd / C. Combine the reaction liquid and washing liquid, rectify and recover ethanol under normal pressure to obtain 210g of light brown transparent viscous liquid, distill at 180-200°C and 1-2mmHg, collect fractions, and obtain light yellow transparent viscous liquid, namely 180.5 g of a mixture of (R,R)-3-(1'-methylbenzylamine)-butanol and (R,S)-3-(1'-methylbenzylamine)-butanol. After detection, RR / RS=82 / 18 in the mixture, 64%ee, reaction yield 93.5%, gas phase content ...
Embodiment 2
[0080] 1) Amination reduction reaction: In a reaction kettle, add 121g (R)-1-methylbenzylamine, 1184g n-hexane, 105.6g butanol and 45g Pt / C catalyst (5%). After sequentially replacing with nitrogen and hydrogen, react at 4MPa pressure and 50°C temperature for 8 hours. GC analysis of the starting material is complete. After the reaction was completed, the Pt / C catalyst was filtered, and the filter cake was rinsed with 160 g of n-hexane, and then vacuum-dried at 40° C. to obtain 45 g of recovered Pt / C. Combine the reaction liquid and washing liquid, rectify at normal pressure to recover n-hexane, obtain 208 g of light brown transparent viscous liquid, distill at 180-200 ° C, 1-2 mmHg, collect fractions, obtain light yellow transparent viscous liquid, That is, 179.5 g of a mixture of (R,R)-3-(1'-methylbenzylamine)-butanol and (R,S)-3-(1'-methylbenzylamine)-butanol. After detection, RR / RS=82 / 18 in the mixture, 64%ee, reaction yield 93.0%, gas phase content 98.6%.
[0081] 2) Re...
Embodiment 3
[0086] 1) Amination reduction reaction: In a reaction kettle, add 121g (R)-1-methylbenzylamine, 1184g n-hexane, 105.6g butanol and 45g Pd / C catalyst (5%). After sequentially replacing with nitrogen and hydrogen, react at 4MPa pressure and 50°C temperature for 8 hours. GC analysis of the starting material is complete. After the reaction was completed, the Pt / C catalyst was filtered, and the filter cake was rinsed with 160 g of n-hexane, and then vacuum-dried at 40° C. to obtain 45 g of recovered Pt / C. Combine the reaction liquid and washing liquid, rectify at normal pressure to recover n-hexane, obtain 208 g of light brown transparent viscous liquid, distill at 180-200 ° C, 1-2 mmHg, collect fractions, obtain light yellow transparent viscous liquid, That is, 181.5 g of a mixture of (R,R)-3-(1'-methylbenzylamine)-butanol and (R,S)-3-(1'-methylbenzylamine)-butanol. After testing, RR / RS=82 / 18 in the mixture, 64%ee, reaction yield 94.0%, gas phase content 98.4%
[0087] 2) Deben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com