Catalytic conversion method of aldose into ketose
A catalytic conversion method and technology of aldulose, applied in the field of catalytic conversion of aldulose, can solve the problems of many by-products, limited substrate concentration and high research and development cost, and achieve the effects of low equipment requirements, simple separation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Reflux the single-walled carbon nanotubes in 0.1M nitric acid solution at 80°C for 4 hours, wash and dry to obtain oxidation-modified single-walled carbon nanotubes, wherein the carboxyl content is 0.5mmol / g, and the phenolic hydroxyl content is 0.1mmol / g, and the lactone content is 0.05mmol / g; using the impregnation method, immerse the oxidation-modified single-walled carbon nanotubes in ferric chloride solution, and introduce 0.1wt % iron element, after drying, an oxidation-modified single-walled carbon nanotube loaded with iron element is obtained.
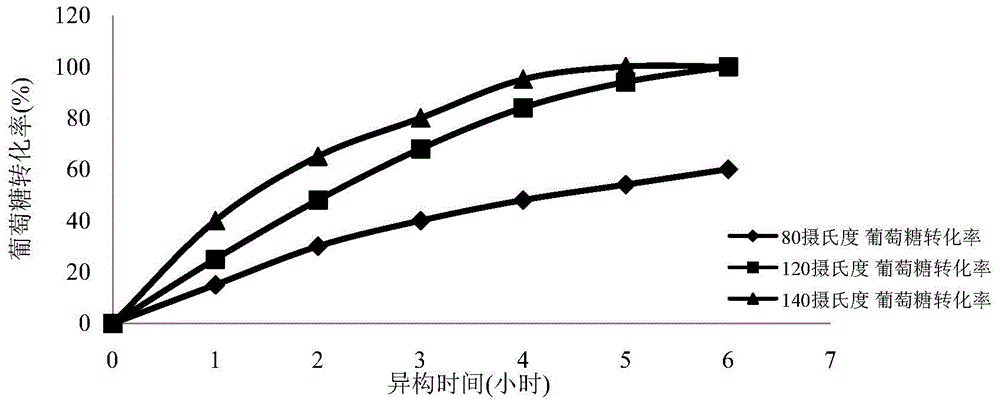
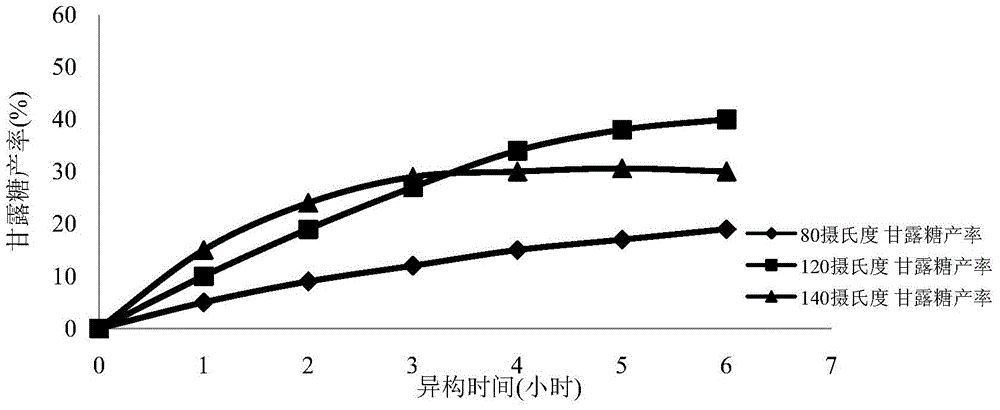
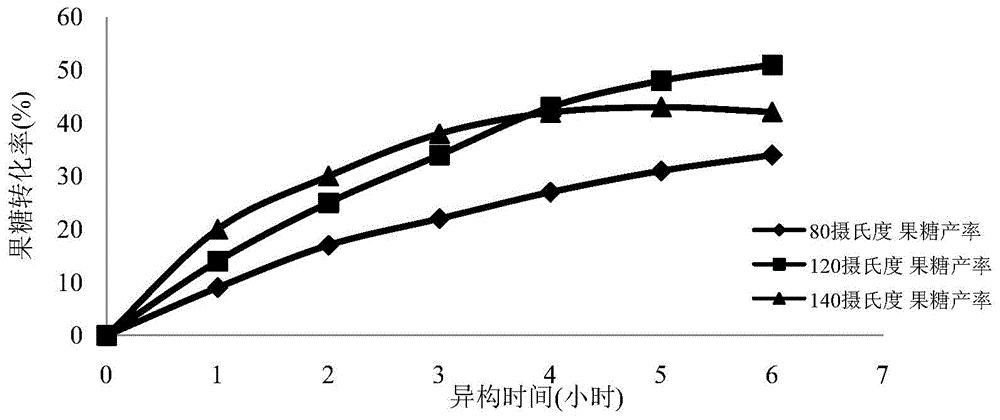
[0055] (2) Weigh 10 g of glucose and add water to 100 ml to prepare a 100 g / L glucose solution. Weighing 2 grams of the oxidation-modified single-walled carbon nanotubes loaded with iron element prepared in step (1), and adding it into the glucose solution to obtain a reaction solution. Divide the reaction solution into 3 parts, 30 ml each, seal and place them in oil baths at 80°C, 120°C, and 140°C, respectively, wi...
Embodiment 2
[0060] (1) Treat multi-walled carbon nanotubes in hydrogen peroxide at room temperature for 4 hours, wash and dry to obtain oxidatively modified multi-walled carbon nanotubes, wherein the carboxyl content is 0.4mmol / g, and the phenolic hydroxyl content is 0.2mmol / g, The content of lactone is 0.03mmol / g; the oxidatively modified multi-walled carbon nanotubes are immersed in manganese nitrate solution by impregnation method, and 0.05wt% manganese element is introduced into the oxidatively modified multi-walled carbon nanotubes, and after drying Oxidatively modified multi-walled carbon nanotubes containing manganese are obtained.
[0061] (2) Weigh 10 grams of arabinose and add water to 200 ml to prepare a 50 g / L arabinose solution. This arabinose solution is divided into 4 bottles, each bottle is 30 milliliters, and 0.5 grams, 1 gram, 1.5 grams, 3 grams of multi-walled carbon nanotubes containing manganese elements obtained in step (1) are added respectively, sealed After that,...
Embodiment 3
[0065] (1) Treat the multi-walled carbon nanotubes with ozone at room temperature for 2 hours to obtain oxidatively modified multi-walled carbon nanotubes, wherein the carboxyl content is 0.8mmol / g, the phenolic hydroxyl content is 0.08mmol / g, and the lactone content is 0.01mmol / g; adopt the impregnation method, immerse the multi-walled carbon nanotubes of oxidation modification in the aluminum sulfate solution, introduce the aluminum element of 0.2wt% in the multi-walled carbon nanotubes of oxidation modification, after drying, obtain containing aluminum element Oxidation-modified multi-walled carbon nanotubes.
[0066] (2) 20 grams of oxidatively modified multi-walled carbon nanotubes containing aluminum elements that step (1) makes are weighed, packed into a fixed-bed reactor (diameter 15mm, long 1000mm), and the temperature of the fixed-bed reactor is raised to To 120 degrees Celsius, the pressure rises to 1 MPa. Weigh 1 kg of galactose and add water to 2.5 liters to for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com