Ginsenoside cyclodextrin inclusion compound and preparation method thereof
A technology of cyclodextrin inclusion compound and ginsenoside, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of application limitations and poor water solubility, and achieve The preparation method is simple and easy, and the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of inclusion compound of ginsenoside F1 and hydroxypropyl-β-cyclodextrin
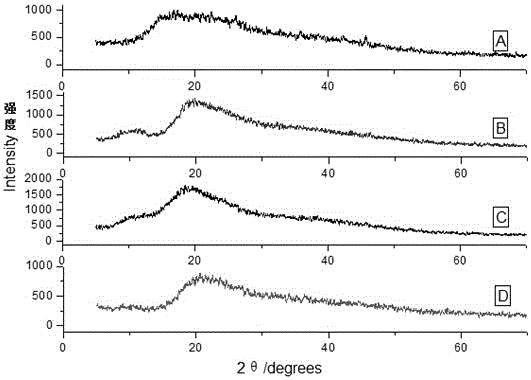
[0028] Add hydroxypropyl-β-cyclodextrin (161.8mg, 0.1mmol) into a 25mL reaction bottle, add 20mL of distilled water and stir until dissolved, then add ginsenoside F1 (63.87mg, 0.1mmol), and protect from light at 25°C Stir for 72 hours, filter to remove insoluble matter, then filter with a 0.45 μm microporous membrane, evaporate the filtrate to dryness to obtain a white powder, and dry in a vacuum oven for 24 hours to obtain the mixture of ginsenoside F1 and hydroxypropyl-β-cyclodextrin Solid clathrates, the clathrates of prepared ginsenosides and cyclodextrins were all characterized by X-ray powder diffraction ( image 3 ), the water solubility comparison before and after inclusion is shown in Table 1.
Embodiment 2
[0029] Example 2: Preparation of inclusion compound of ginsenoside F1 and sulfobutyl ether-β-cyclodextrin
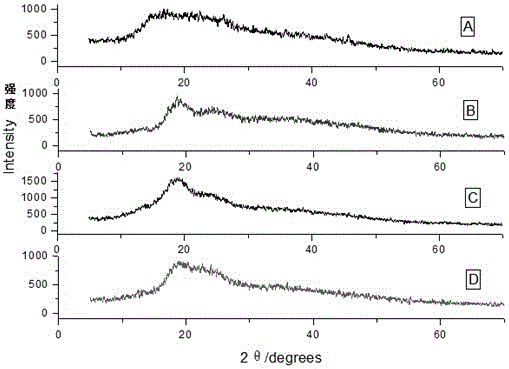
[0030] Add sulfobutyl ether-β-cyclodextrin (700mg, 0.5mmol) into a 25mL reaction bottle and add 20mL of distilled water and stir until dissolved, then add ginsenoside F1 (63.87mg, 0.1mmol), and protect from light at 25°C Ultrasound for 10 hours, filter to remove insoluble matter, then filter with a 0.45 μm microporous membrane, evaporate the filtrate to dryness to obtain a white powder, dry in a vacuum oven for 24 hours, and obtain ginsenoside F1 and sulfobutyl ether-β-cyclodextrin The solid clathrate of the obtained ginsenoside and the clathrate of cyclodextrin are all characterized by X-ray powder diffraction ( Figure 4 ), the water solubility comparison before and after inclusion is shown in Table 1.
Embodiment 3
[0031] Example 3: Preparation of inclusion compound of ginsenoside Re and hydroxypropyl-β-cyclodextrin
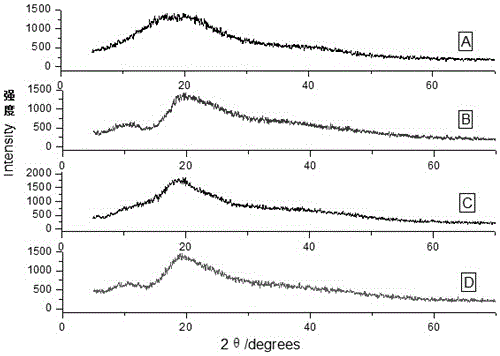
[0032]Add hydroxypropyl-β-cyclodextrin (1132.6mg, 0.7mmol) into a 25mL reaction bottle, add 20mL of distilled water and stir until dissolved, then add ginsenoside Re (94.72mg, 0.1mmol), and protect from light at 60°C Sonicate for 1 hour, filter to remove insoluble matter, then filter with a 0.45 μm microporous membrane, evaporate the filtrate to dryness to obtain a white powder, and dry it in a vacuum oven for 24 hours to obtain the mixture of ginsenoside Re and hydroxypropyl-β-cyclodextrin Solid clathrates, the clathrates of prepared ginsenosides and cyclodextrins were all characterized by X-ray powder diffraction ( Figure 7 ), the water solubility comparison before and after inclusion is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com