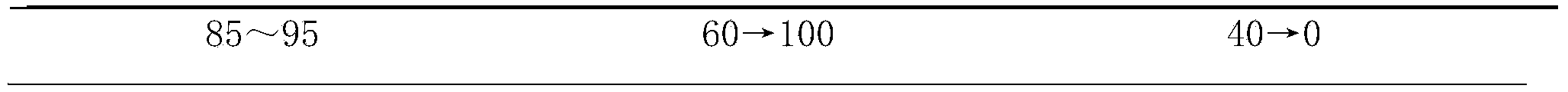Ginsenoside-ophiopogonin D composition for treating cardiovascular and cerebrovascular diseases
A cardiovascular and cerebrovascular disease, ginsenoside technology, applied in the field of medicine, can solve the problems of increasing the risk of adverse reactions of patients, uncontrolled mass ratio, and increased incidence of adverse reactions, so as to achieve hemolysis and coagulation safety and improve safety and effectiveness, the effect of component fixation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
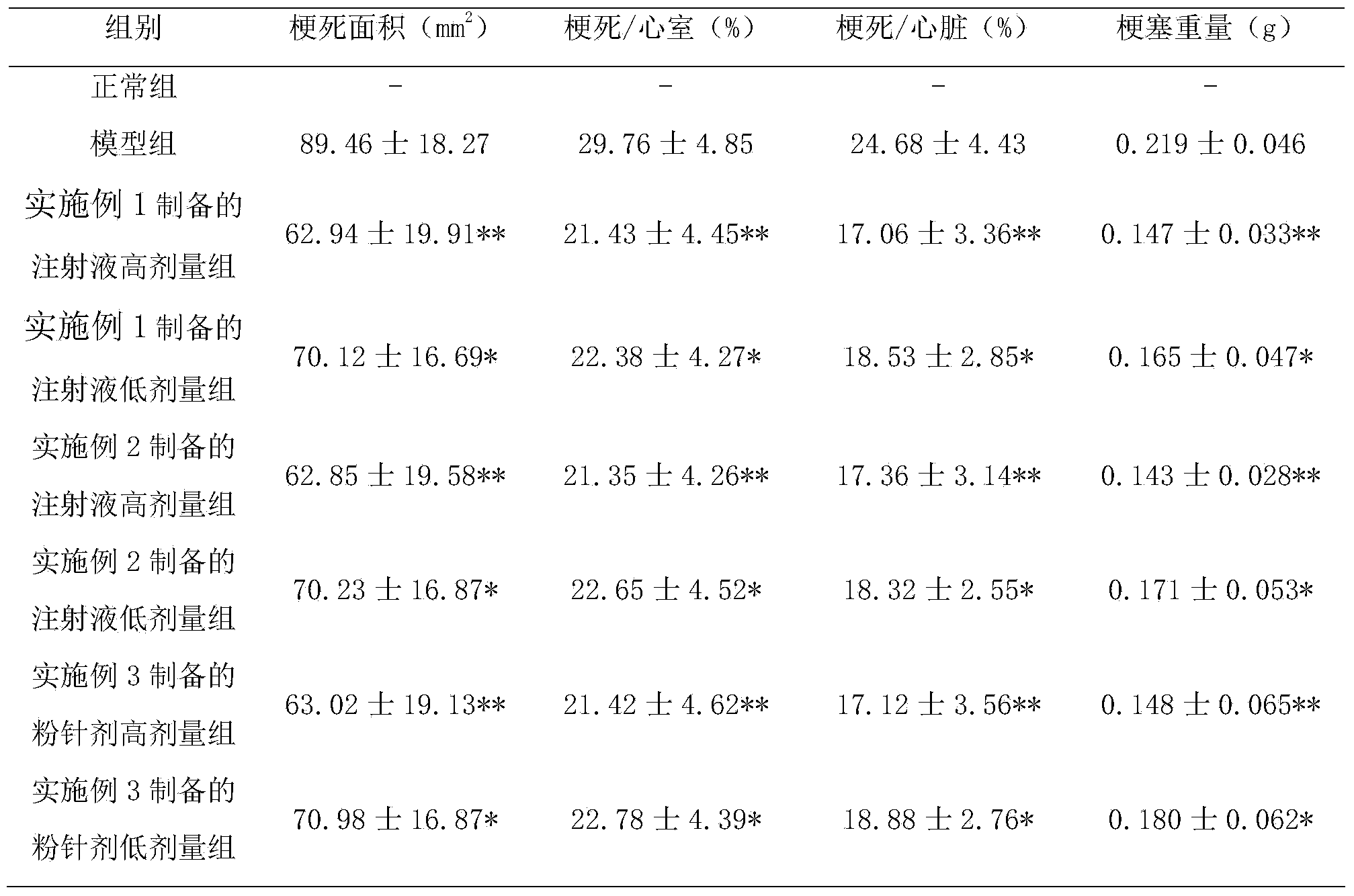
Examples
Embodiment 1
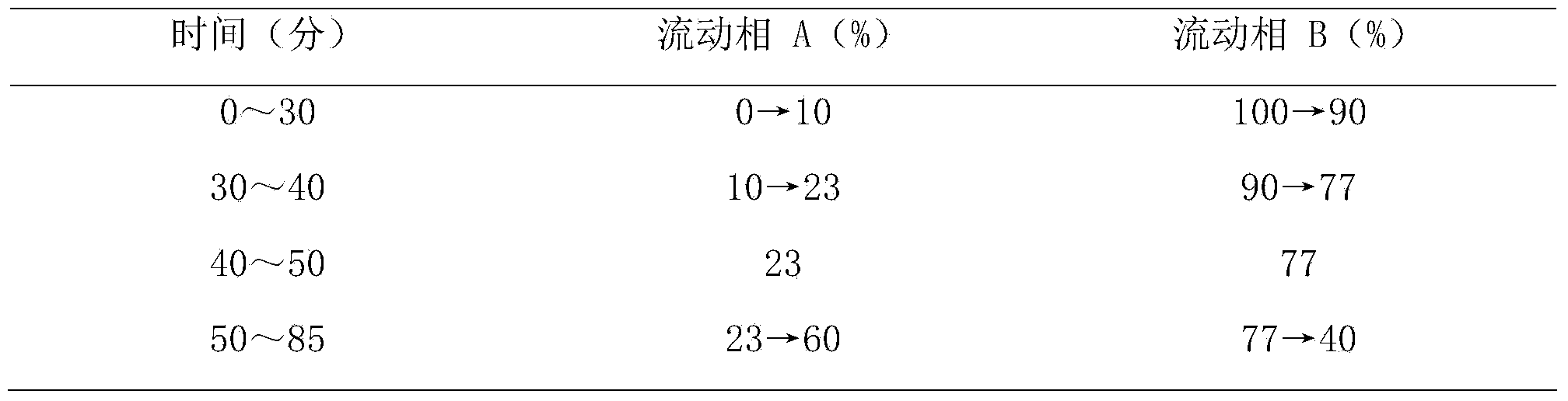
[0039] Embodiment 1 prepares the injection of composition of the present invention
[0040] The sample prepared by the above-mentioned steps is detected by the above-mentioned method again, and the purity is respectively ginsenoside Rb 1 99.6%, Ginsenoside Rg 1 99.5%, Ginsenoside Rb 2 99.0%, ginsenoside Rc99.2%, ginsenoside Re99.3%, ophiopogon saponin D99.4%.
[0041] Ginsenoside Rb 1 32%, Ginsenoside Rg 1 18%, Ginsenoside Rb 2 14%, ginsenoside Rc14%, ginsenoside Re8%, ophiopogon saponin D14%, add appropriate amount of water for injection to dissolve, ultrafiltration, potting, and make injection.
Embodiment 2
[0042] Embodiment 2 prepares the injection of composition of the present invention
[0043] The sample prepared by the above-mentioned steps is detected by the above-mentioned method again, and the purity is respectively ginsenoside Rb 1 91.3%, Ginsenoside Rg 1 92.1%, ginsenoside Rb 2 90.0%, ginsenoside Rc91.5%, ginsenoside Re90.8%, ophiopogon saponin D91.4%.
[0044] Ginsenoside Rb 1 28%, Ginsenoside Rg 1 20%, Ginsenoside Rb 2 14%, ginsenoside Rc14%, ginsenoside Re10%, ophiopogon saponin D14%, add appropriate amount of water for injection to dissolve, then add appropriate amount of activated carbon, filter, pot, and make injection.
Embodiment 3
[0045] Embodiment 3 prepares the lyophilized powder injection of composition of the present invention
[0046] The sample prepared by the above-mentioned steps is detected by the above-mentioned method again, and the purity is respectively ginsenoside Rb 1 85.6%, Ginsenoside Rg 1 86.2%, ginsenoside Rb 2 85.1%, ginsenoside Rc85.8%, ginsenoside Re85.3%, ophiopogon saponin D86.0%.
[0047] Ginsenoside Rb 1 28%, Ginsenoside Rg 1 22%, Ginsenoside Rb 2 14%, ginsenoside Rc14%, ginsenoside Re8%, ophiopogon saponin D14%, add appropriate amount of water for injection to dissolve, filter, potting, and make freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com