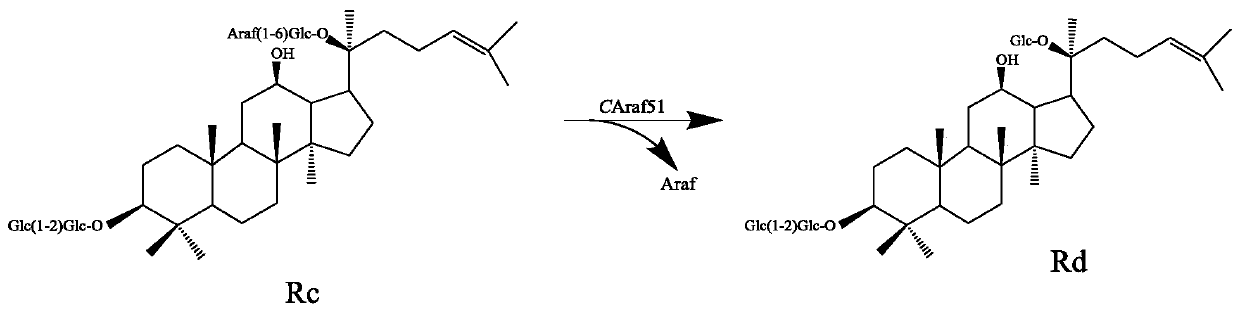
Alpha-L-arabinfuranosidease and coding gene and application thereof
A furanosidase and gene technology, applied in the fields of genetic engineering technology and biomedicine, can solve the problems of poor sugar tolerance, low Rd yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1) Acquisition of genes
[0064] According to the nucleotide sequence shown in SEQ ID NO: 2, the corresponding gene was obtained by artificial chemical synthesis (entrusted to Kunming Shuoqing Biotechnology Co., Ltd.).
[0065] (2) Construction of expression vectors and recombinant strains
[0066] Both the gene obtained in step (1) and the expression vector pET28a (+) were subjected to EcoRI / HindIII double enzyme digestion, and after the digestion product was purified by nucleic acid, DNA ligase was used to connect the above two digested products (16 ℃ overnight reaction) to obtain a recombinant plasmid, the sequence of which is shown in SEQ ID NO: 2 after restriction digestion and sequencing verification.
[0067] The obtained recombinant plasmid was transformed into Escherichia coli BL21 to obtain a recombinant strain (BL21-pET28a-CaAraf51).
Embodiment 2
[0069]The recombinant strain (BL21-pET28a-CaAraf51) constructed in Example 1 was inoculated in 10 ml of LB liquid medium, and cultivated overnight at 180 rpm at 37°C for about 13 hours; the next day, it was inoculated into 100 ml of fresh In LB medium, until OD 600 When it was 0.8, IPTG with a final concentration of 0.5 mM was added, and cultured at 180 rpm and 25° C. for 15 h.
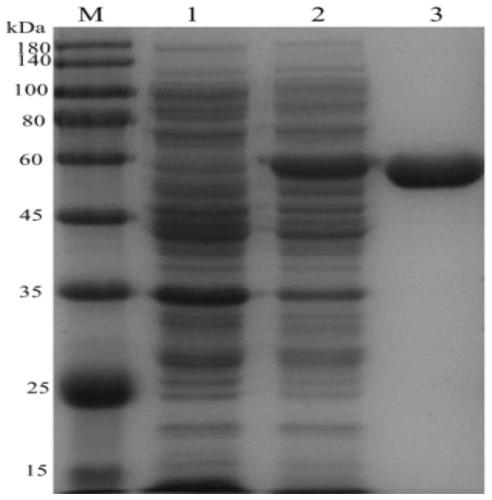
[0070] Preparation of crude protein solution: collect the cells by centrifugation at 9000rpm, 4°C for 5 minutes, pour off the supernatant; wash the cells twice with PB buffer, and finally resuspend the cells in 20ml equilibration buffer; ultrasonically break the cells (working 4s, Pause for 4s, amplitude 40%) for 30min; 12000rpm, centrifuge at 4°C for 20min to collect the supernatant (crude protein solution) for SDS-PAGE analysis; the supernatant contains the protein expressed by the target gene, ie crude protein.
[0071] Protein purification: filter the crude protein solution with a 0.45mm micropor...
Embodiment 3
[0074] Qualitative determination of α-L-arabinofuranosidase (CaAraf51) of the present invention
[0075] 1. Determination method of enzyme activity
[0076] Add the substrate (p-nitrophenyl-α-L-arabinofuranoside) to 100 μl reaction system to make the final concentration 5 mM, then add 5 μl purified enzyme solution, pH7.5 (phosphate buffer), 37 ° C React under the reaction conditions for 10min, then immediately add 100μl, 1M Na 2 CO 3 , Measure the absorbance of the reaction product p-nitrophenol (pNP) at 405 nm.
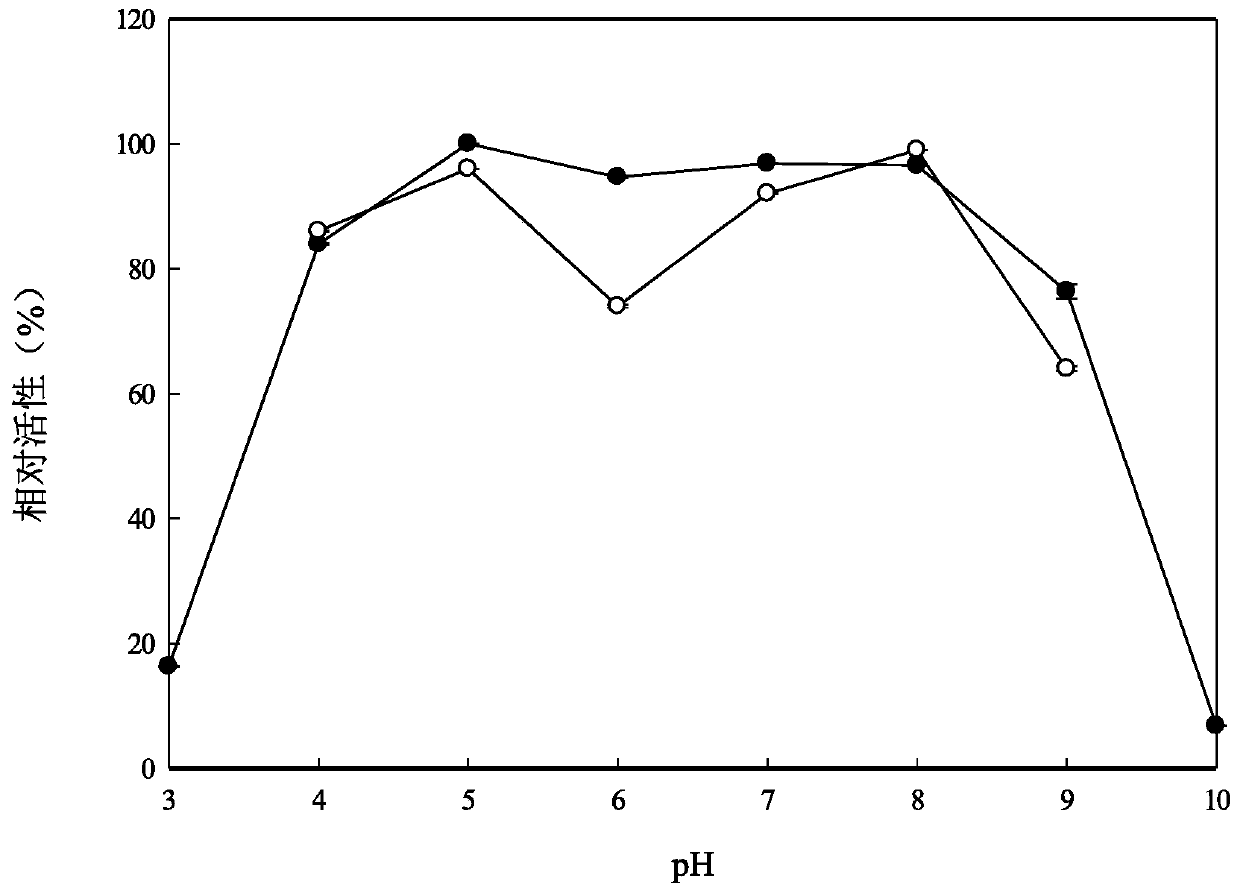
[0077] 2. Determination of optimum pH and pH stability
[0078] Determination of pH optimum and pH stability was performed using the following buffers: 50 mM glycine-HCl (pH 2 and 3), 50 mM acetic acid-sodium acetate (pH 4 and 5), 50 mM Na 2 HPO 4 -NaH 2 PO 4 (pH 6-8), 50 mM Glycine-NaOH (pH 9 and 10).
[0079] Add the substrate to 100 μl of buffer solution with different pH to make the final concentration 5mM, then add 5 μl of purified enzyme solution, incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com