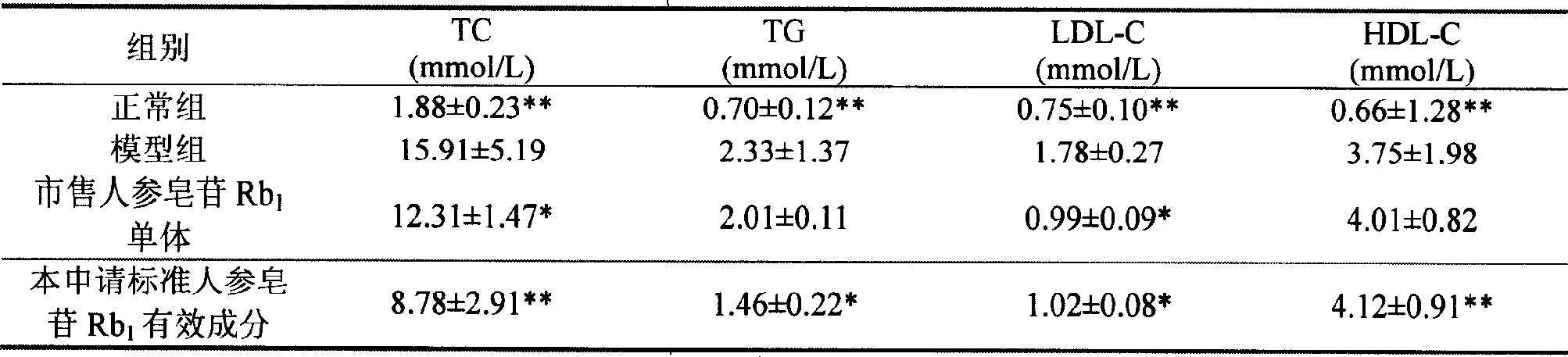
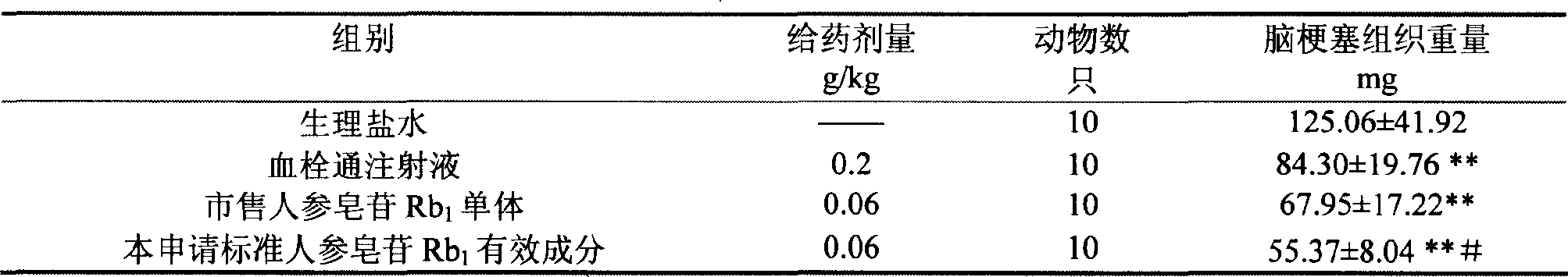
Ginsenoside Rb1 containing impurity ginsenoside Rc
A ginsenoside content technology, which is applied to medical preparations containing active ingredients, organic active ingredients, plant raw materials, etc., can solve the problems of no content measurement, high cost, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
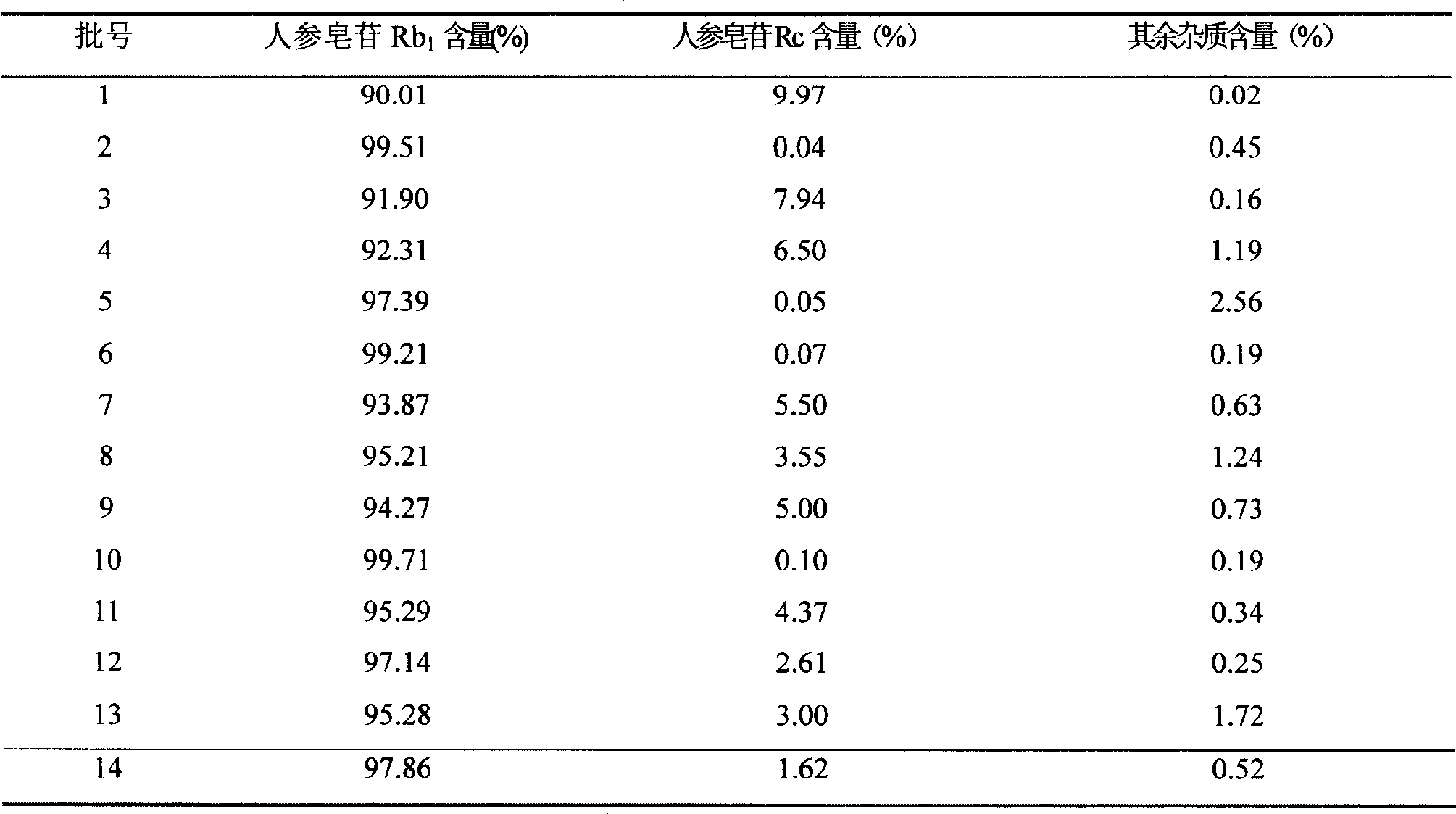
[0107] A ginsenoside Rb 1 , Ginsenoside Rb 1 The content is 90.02%, the impurity content is 9.98%, the impurity includes ginsenoside Rc, and the ginsenoside Rc content is 9.95.
[0108] The above ginsenoside as Rb 1 Pharmaceutical raw materials can be prepared into pharmaceutical preparations acceptable to pharmaceutics.
Embodiment 2
[0110] A ginsenoside Rb 1 , Ginsenoside Rb 1 The content is 99.80%, the impurity content is 0.20%, the impurity includes ginsenoside Rc, and the content of ginsenoside Rc is 0.13%.
[0111] The above ginsenoside as Rb 1 Pharmaceutical raw materials can be prepared into pharmaceutical preparations acceptable to pharmaceutics.
Embodiment 3
[0113] A ginsenoside Rb 1 , Ginsenoside Rb 1 The content is 91.82%, the impurity content is 8.18%, the impurity includes ginsenoside Rc, and the content of ginsenoside Rc is 8.09%.
[0114] The above ginsenoside as Rb 1 Pharmaceutical raw materials can be prepared into pharmaceutical preparations acceptable to pharmaceutics.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com