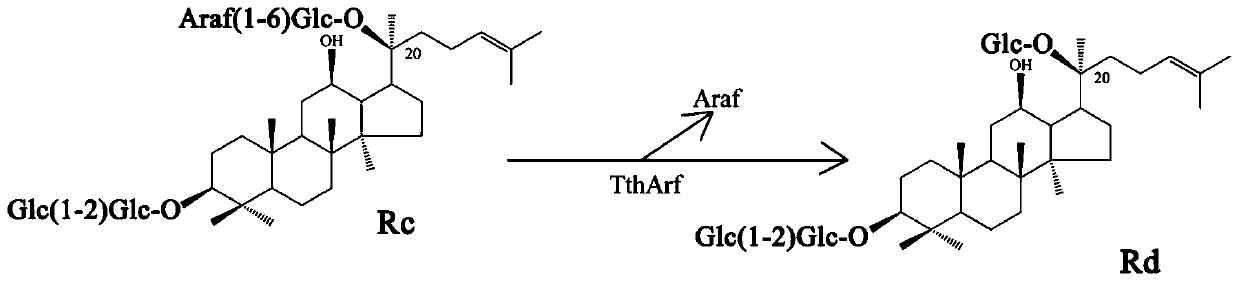
α-l-arabinofuranosidase and its application in the preparation of ginsenoside rd
The technology of furanosidase and ginsenoside is applied in the application field of α-L-arabinofuranosidase, enzymatically converting multi-component ginsenoside Rc to prepare ginsenoside Rd, and achieves the effects of high tolerance and strong transformation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: The acquisition of the α-L-arabinofuranosidase gene of the present invention and the construction of the recombinant plasmid pET-TthArf
[0040] 1.1 Thermotoga thermarum Cultivation of DSM 5069
[0041] Thermotoga thermarum DSM 5069 was purchased from the DSMZ Culture Collection Center (www.dsmz.de) with the number 5069, and its medium formula was: 10 g / L starch, 5 g / L tryptone, 3 g / L yeast extract, 5 g / L L meat extract, 10g / L 2-morpholineethanesulfonic acid, 10 mg / L ferric sulfate heptahydrate, 1 mg / L resazurin, adjust the pH to 7.2. Inoculate with a syringe according to the inoculum volume of 0.5%, culture statically at 85°C for 24 h, and collect the cells.
[0042] 1.2 Extraction of genomic DNA
[0043] (1) static culture Thermotoga thermarum DSM 5069 for about 24 hours, take 30 mL of the bacterial liquid and centrifuge at 4,000 g for 10 min to collect the cells.
[0044] (2) Resuspend the cells in 9.5 mL TE buffer, add 0.5 mL 10% sodium dod...
Embodiment 2
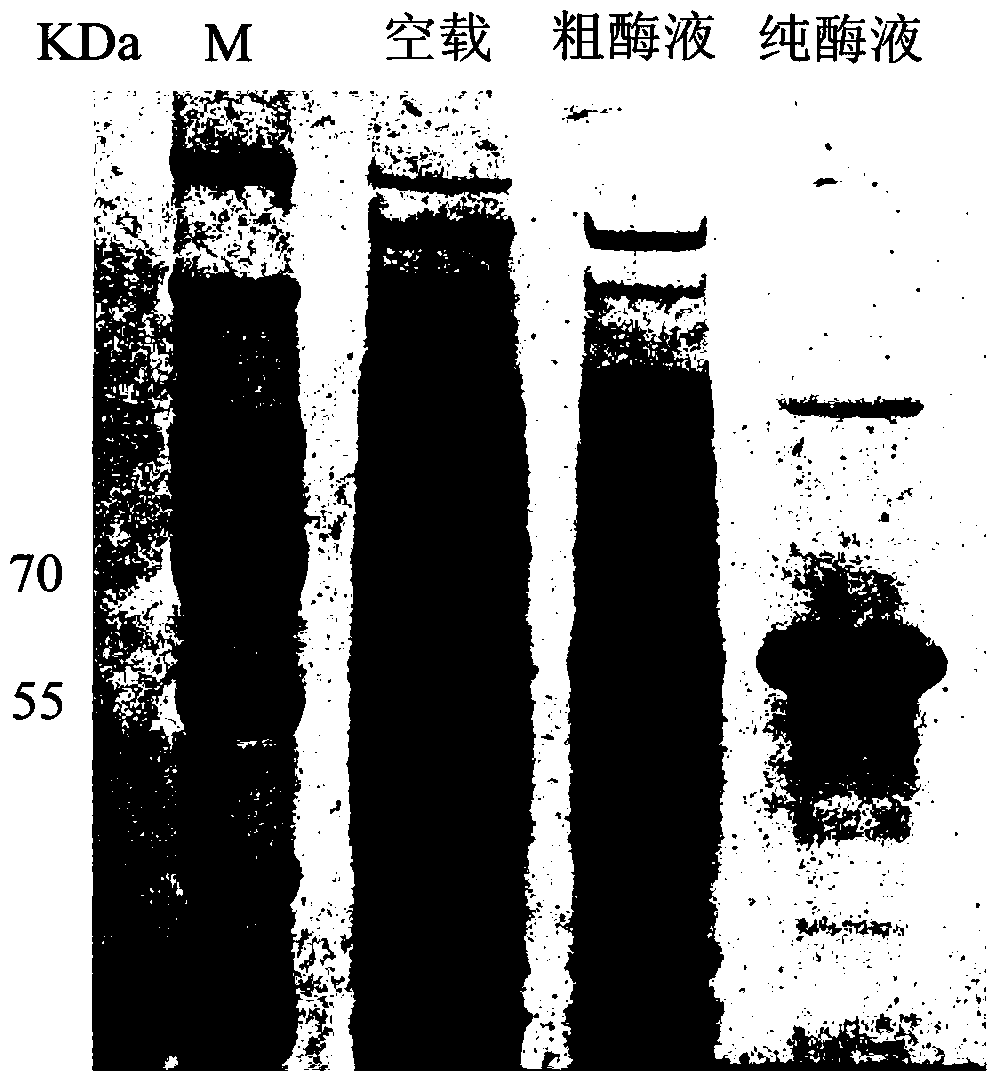
[0059] Embodiment 2: Preparation of α-L-arabinofuranosidase TthArf of the present invention
[0060] The recombinant plasmid pET-TthArf was transformed into Escherichia coli JM109(DE3) host bacteria (purchased from Novagen), on LB plates containing kanamycin (50 μg / mL) (LB medium: tryptone 10 g / L, yeast Extract 5 g / L, NaCl 5 g / L, agar 15 g / L) after culturing overnight at 37°C, pick the transformant into 200 mL of LB medium (50 μg / mL kanamycin) at 37°C, 200 Shake the culture at rpm until the OD600 is 0.6, add a final concentration of 0.5 mM isopropyl β-D-thiogalactopyranoside (IPTG) inducer, culture at 30 ° C for 8 h, and use a high-speed refrigerated centrifuge to distill the culture solution Centrifuge at 13,000 rpm for 15 min at 4°C to collect the cells.
[0061] Since the recombinant plasmid pET-TthArf contains a His-tag tag, it was purified by His•Bind Purification Kit (purchased from Novagen) to obtain a purified recombinant enzyme. The specific operation process:
[0...
Embodiment 3
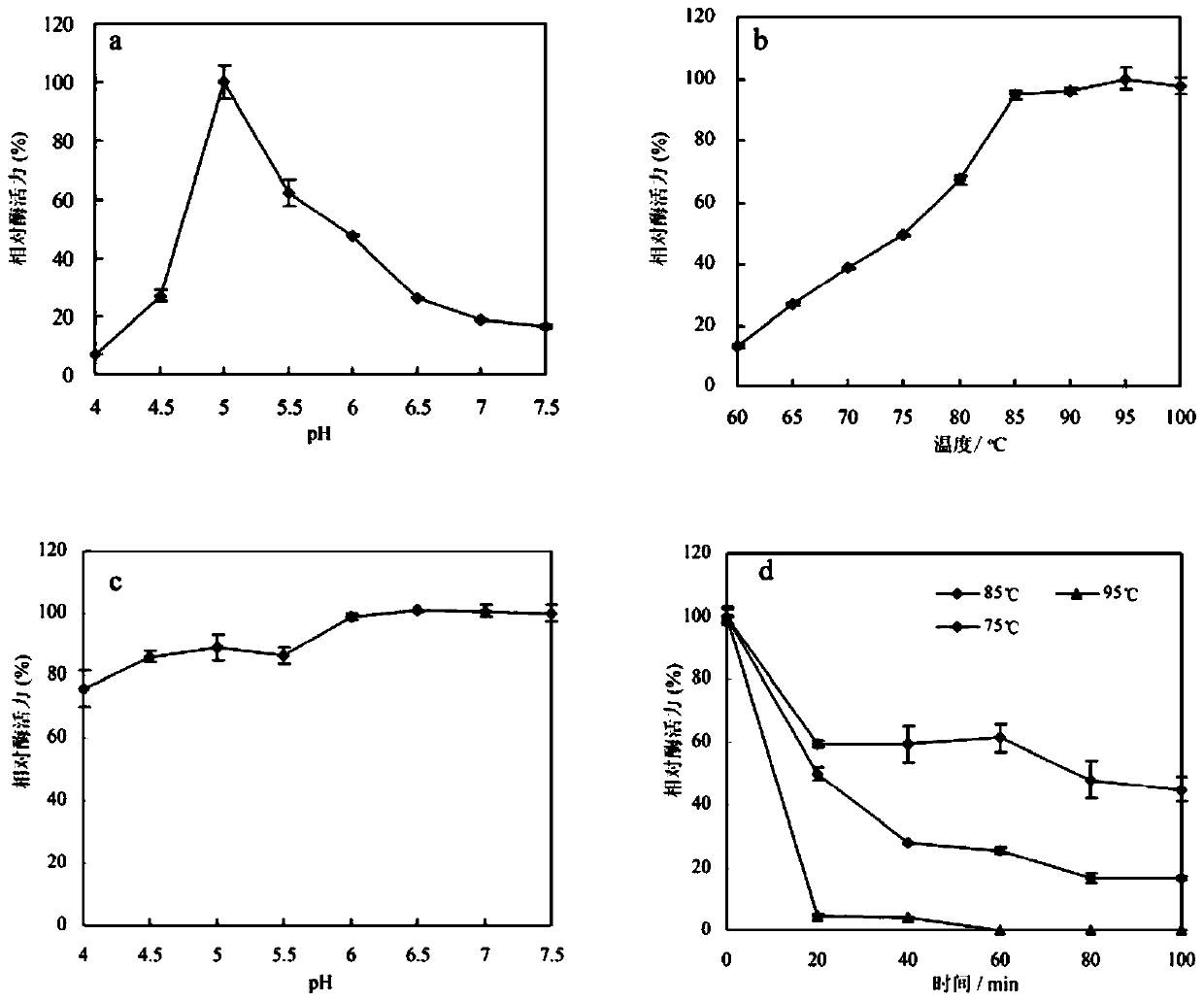
[0078] Embodiment 3: Qualitative determination of α-L-arabinofuranosidase TthArf of the present invention
[0079] 1. Determination method of enzyme activity
[0080] Reaction system 100 μL, 5 μL 20 mmol / L p-nitrophenyl α-D-arabinofuranoside ( p NPArf) was added to 85 μL 100 mmol / L citric acid-disodium hydrogen phosphate buffer (pH 6.0), first at 90 o C for 3 min, then add 10 μL of enzyme solution (diluted to an appropriate concentration) and react for 10 min, after color development, add 600 μL of 1 mol / L sodium carbonate solution to terminate the reaction. Absorbance was measured at 405 nm. Enzyme activity unit (U) is defined as: under the assay conditions, the amount of enzyme required to produce 1 μmol p-nitrophenol per minute is 1 enzyme activity unit.
[0081] 2. Determination of the optimum reaction pH
[0082] Under the conditions of different pH (3.0-7.5, 100 mmol / L citric acid-disodium hydrogen phosphate buffer solution), the enzyme activity was measured at 90°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com