Method for determining content of ginsenoside in panax traditional Chinese medicine
A technology of ginsenosides and pseudo-ginsenosides, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of difficult ginsenoside component detection, few index components, weak specificity, etc., and achieves perfect quality standards and strong specificity. , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
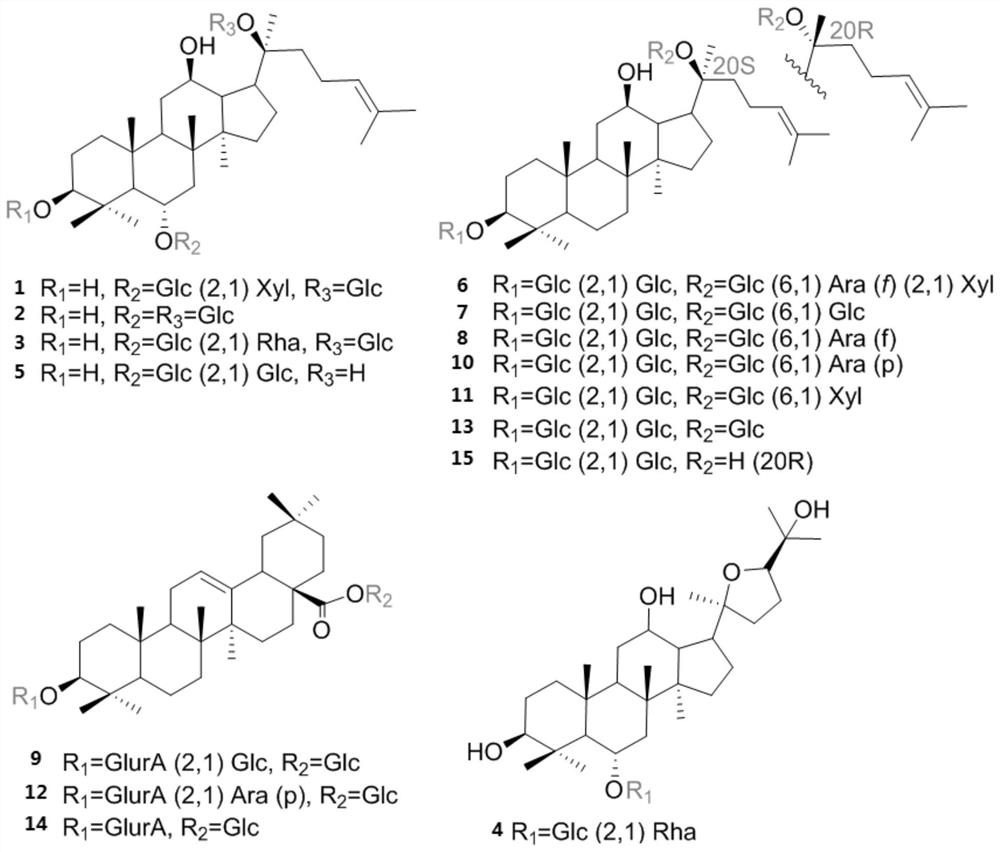
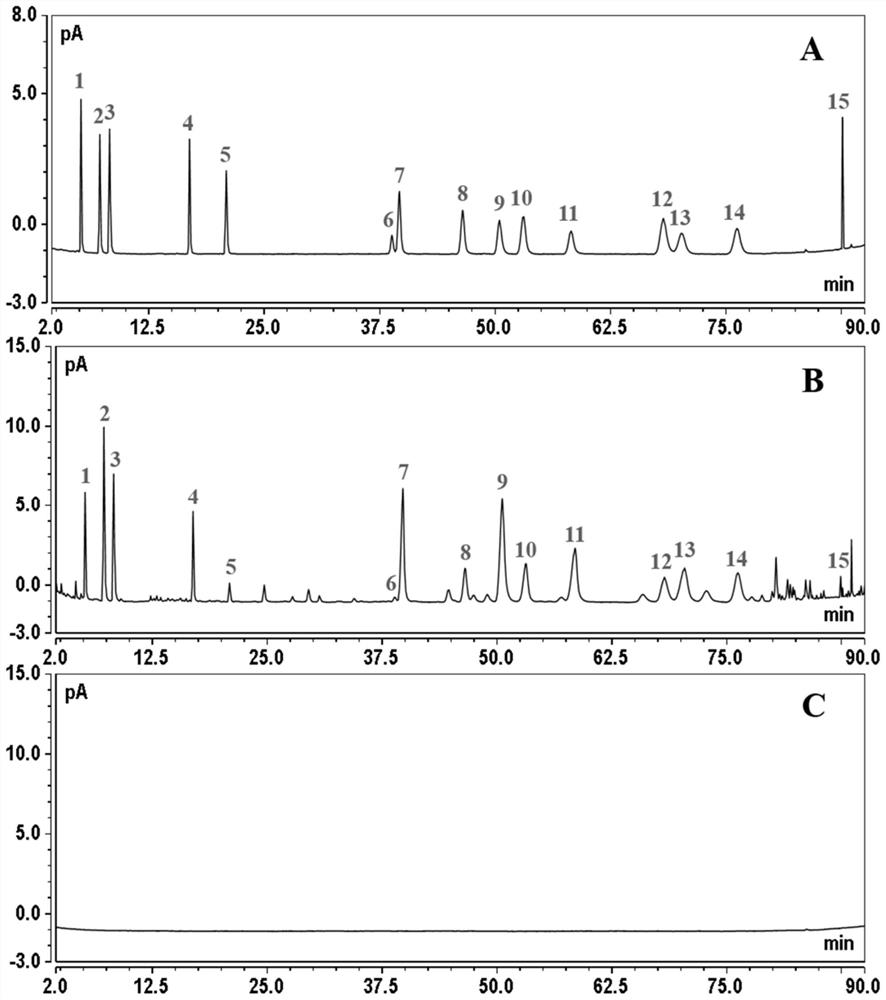
[0045] By adopting the preparation method of the sample solution to be tested in the present application, the sample solution to be tested including 15 kinds of ginsenosides can be obtained, so that the content detection results of the ginsenoside components in the sample to be tested are more comprehensive, accurate and credible.
[0046] In some embodiments of the present application, the chromatographic column is selected from any one of CORTECS UPLC Shield RP18, Kinetex EVO C18, BEH Shield RP18 or Atlantis Premier BEH C18 AX.
[0047] The inventors found in the research that using the gradient elution of the present application, the 15 kinds of ginsenosides can be separated better. Preferably, in some embodiments of the present application, the gradient elution is specifically: 0-7 minutes, 20% B; 7-9 minutes, 20-24% B; 9-32 minutes, 24-26% B; 32-72 minutes, 26-26% B; 72-80 minutes, 26-35 %B; 80-86 minutes, 35-50%B; 86-92 minutes, 50-60%B; 92-95 minutes, 60-98%B; 95-98 min...
Embodiment 1
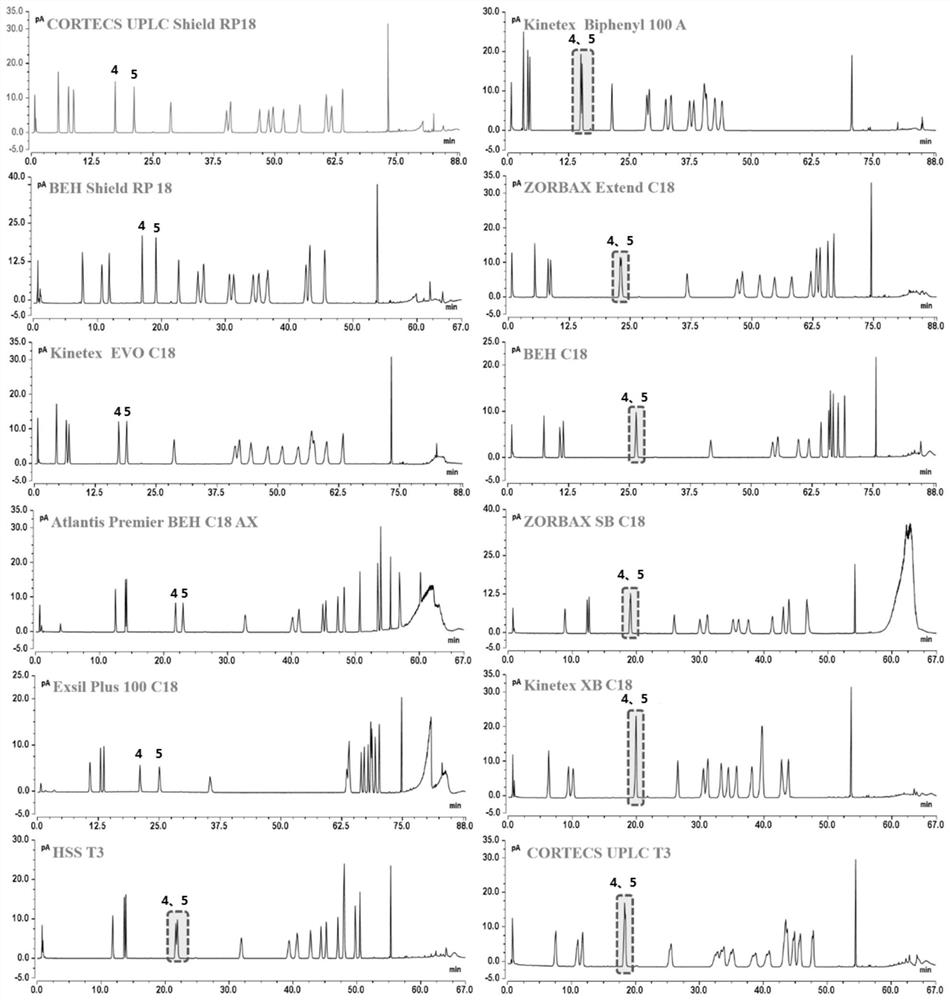
[0065] Embodiment 1 chromatographic column is determined
[0066] Chromatographic conditions: select 12 chromatographic columns as shown in Table 3 for the chromatographic columns respectively.
[0067] Mobile phase: Phase A is 0.1% formic acid aqueous solution, phase B is acetonitrile; column temperature: 30°C; flow rate: 0.3mL / min; injection volume: 3μL; gradient elution: according to the different chromatographic columns in Table 3 for ginsenoside Two gradients were used to determine the different retention capacities.
[0068] Among them, when using the chromatographic columns with the numbers 1, 3, 5, and 7-9 in Table 3, the elution gradient: 0-7min, 20% B; 7-9min, 20-24% B; 9-24min, 24% -27%B; 24-50min, 27-27%B; 50-58min, 27-28%B; 58-62min, 28-32%B; 62-66min, 32-35%B; 66-71min, 35% -50%B; 71-75min, 50-65%B; 75-78min, 65-98%B; 78-81min, 98%B;
[0069]When using the chromatographic columns numbered 2, 4, 6, and 10-12 in Table 3, the elution gradient: 0-7min, 20% B; 7-11...
Embodiment 2
[0073] Embodiment 2 column temperature is determined
[0074] Chromatographic conditions: select the column temperature as 25°C, 28°C, 30°C, 35°C, and 40°C respectively.
[0075] Chromatographic column: CORTECS UPLC Shield RP18; mobile phase: phase A is 0.1% formic acid aqueous solution, phase B is acetonitrile; flow rate: 0.3mL / min; injection volume: 3μL; gradient elution: 0-7min, 20% B; 7 -9min, 20-24%B; 9-24min, 24-27%B; 24-50min, 27-27%B; 50-58min, 27-28%B; 58-62min, 28-32%B; 62 -66min, 32-35%B; 66-71min, 35-50%B; 71-75min, 50-65%B; 75-78min, 65-98%B; 78-81min, 98%B.
[0076] Detection conditions: atomization temperature: 40°C; data acquisition frequency: 5Hz; filter constant: 3.6s; power function: 1.00; gain: 100pA.
[0077] Preparation of the ginseng sample solution to be tested: take 1 g of the sample numbered PG-1 in Table 2, grind and pulverize, accurately weigh 100 mg into a 15 mL centrifuge tube, add 3 mL of 70% methanol aqueous solution for ultrasonic extraction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com