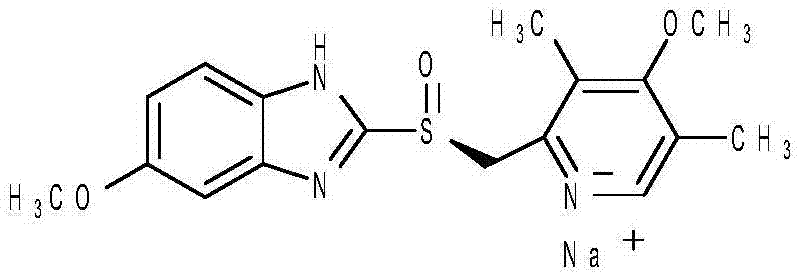
Esomeprazole sodium and method for detecting impurity content in esomeprazole sodium for injection
A technology for esomeprazole sodium and a detection method is applied in the field of drug analysis, and can solve the problems of difficulty in evaluating the quality of esomeprazole, increasing adverse drug reactions, monitoring important impurities, and the like, Achieve the effect of good control, accurate quality control methods, and scientific detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
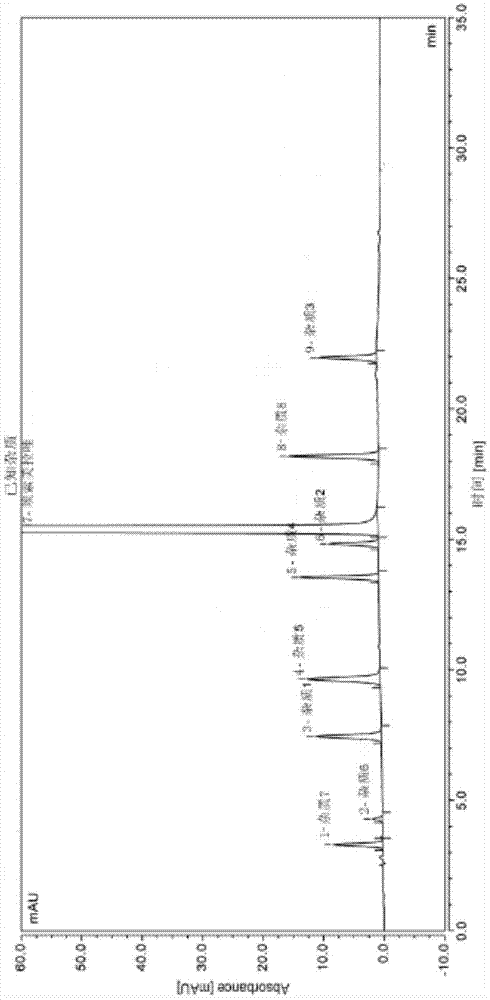
[0026] Embodiment 1 Determination of impurities in esomeprazole sodium by high performance liquid chromatography
[0027] Determination according to high performance liquid phase method (Chinese Pharmacopoeia 2010 edition two appendix V D)
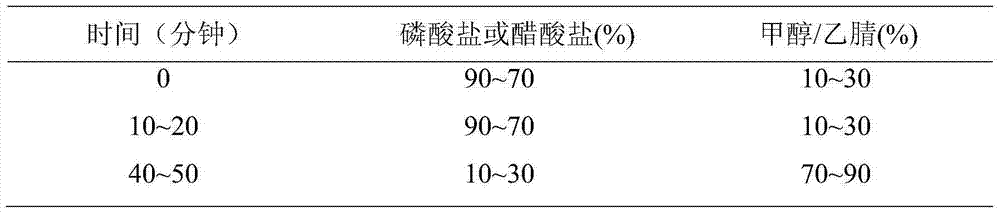
[0028] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler. Mobile phase: mobile phase A is an aqueous solution containing 1.4 g of disodium hydrogen phosphate dodecahydrate per 1 L, adjusted pH to 7.4 with phosphoric acid, mobile phase B is acetonitrile, and performs gradient elution. Take esomeprazole sodium and impurity 4 respectively and dilute with the initial mobile phase to make a mixed solution with a concentration of 0.02mg / ml, as a system suitability solution. Take 10 μl of the system suitability solution and inject it into the liquid chromatograph, the peak order is impurity 4, esomeprazole, and the separation degree of impurity 4 and esomeprazole should meet the requireme...
Embodiment 2
[0036] Embodiment 2 Determination of impurities in esomeprazole sodium by high performance liquid chromatography
[0037] Determination according to high performance liquid phase method (Chinese Pharmacopoeia 2010 edition two appendix V D)
[0038] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler. Mobile phase: mobile phase A is an aqueous solution containing 1.4 g of disodium hydrogen phosphate dodecahydrate per 1 L, adjusted pH to 7.6 with phosphoric acid, mobile phase B is methanol, and gradient elution is performed. Take esomeprazole sodium and impurity 4 respectively and dilute with the initial mobile phase to make a mixed solution with a concentration of 0.02mg / ml, as a system suitability solution. Take 10 μl of the system suitability solution and inject it into the liquid chromatograph, the peak order is impurity 4, esomeprazole, and the separation degree of impurity 4 and esomeprazole should meet the requireme...
Embodiment 3
[0046] Embodiment 3 Determination of impurities in esomeprazole sodium by high performance liquid chromatography
[0047] Determination according to high performance liquid phase method (Chinese Pharmacopoeia 2010 edition two appendix V D)
[0048] Chromatographic conditions and system suitability test Octadecylsilane bonded silica gel was used as filler. Mobile phase: mobile phase A is an aqueous solution containing 1.4 g of disodium hydrogen phosphate dodecahydrate per 1 L, adjusted pH to 7.8 with phosphoric acid, mobile phase B is acetonitrile, and performs gradient elution. Take esomeprazole sodium and impurity 4 respectively and dilute with the initial mobile phase to make a mixed solution with a concentration of 0.02mg / ml, as a system suitability solution. Take 10 μl of the system suitability solution and inject it into the liquid chromatograph, the peak order is impurity 4, esomeprazole, and the separation degree of impurity 4 and esomeprazole should meet the requireme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com