Content detection method for compound houttuynia cordata mixture
A compound Houttuynia cordata and detection method technology, which is applied in the fields of pharmacy and analytical chemistry, can solve the problems of quercitrin and baicalin loss and non-detection, and achieve the effects of ensuring clinical efficacy, simple operation, and eliminating carbohydrate interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, chromatographic column investigation
[0059] Column type:
[0060] 1#: Agilent ZORBAX SB-C18, the column length is 250mm, the inner diameter is 4.6mm, and the packing particle size is 5μm;
[0061] 2#: Agilent ZORBAX Eclipse XDB-C18, the column length is 250mm, the inner diameter is 4.6mm, and the packing particle size is 5μm;
[0062] 3#: Agilent ZORBAX SB-Phenyl, the column length is 250mm, the inner diameter is 4.6mm, and the packing particle size is 5μm;
[0063] A, preparation of reference substance solution:
[0064] Take quercetin, baicalin, and forsythin reference substances respectively, use methanol as solvent, dilute and prepare a mixed standard solution, and prepare a concentration of 0.016mg / ml quercetin, 0.45mg / ml baicalin, 0.020mg / ml ml forsythin reference substance solution;
[0065] B. Preparation of the test solution:
[0066] Precisely draw 25.0ml of the test product, and shake it for 3 times with water-saturated n-butanol, 25ml ea...
Embodiment 2
[0070] Embodiment 2, chromatographic condition investigation
[0071] The preparation method of reference substance solution and need testing solution is with embodiment 1, and detection condition is as follows:
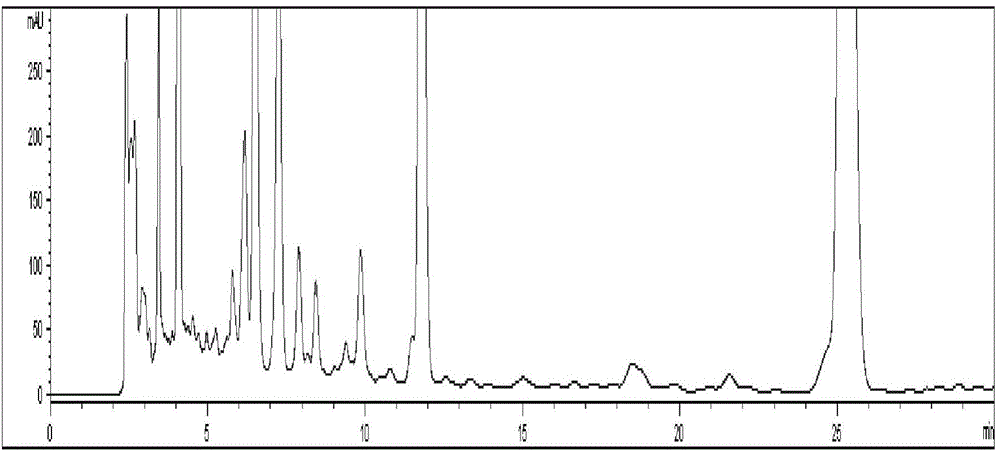
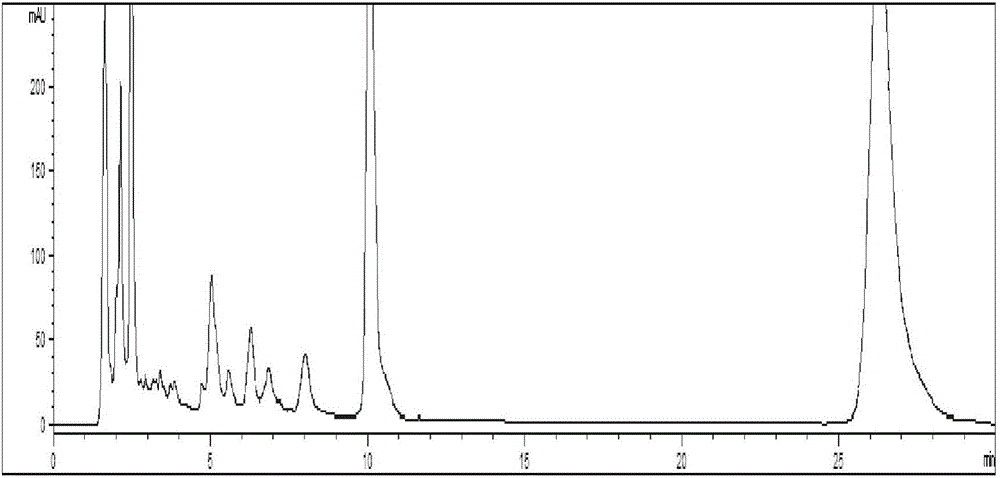
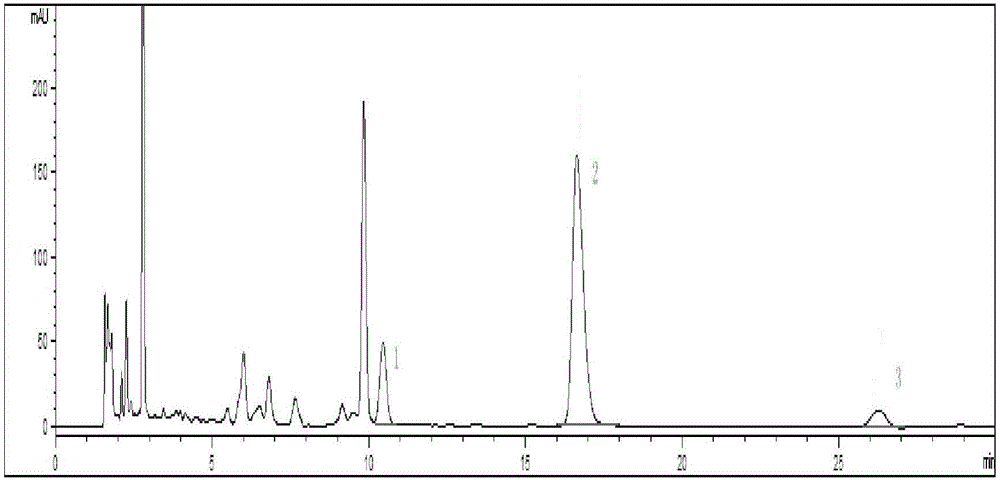
[0072] Chromatographic condition 1: Refer to "Simultaneous Determination of Chlorogenic Acid, Forsythin and Baicalin in Compound Houttuynia Tablets by RP-HPLC", with octadecylsilane bonded silica gel as filler and acetonitrile as mobile phase A , 0.002% phosphoric acid solution was the mobile phase B, and carried out gradient elution according to the elution conditions in Table 1; the flow rate was 1.0ml / min and the detection wavelength was 280nm; 10 μL of the reference substance solution and the test solution were injected into the liquid chromatograph for determination , that is. see attached results Figure 4 .
[0073] Table 1 Elution conditions
[0074] time (min)
Mobile phase A(%)
Mobile phase B(%)
0
10
90
6
30
...
Embodiment 3
[0078] Embodiment 3, need testing solution preparation method-extraction solvent, consumption, frequency investigation
[0079] A, preparation of reference substance solution:
[0080] Take quercetin, baicalin, and forsythin reference substances respectively, use methanol as solvent, dilute and prepare a mixed standard solution, and prepare a concentration of 0.016mg / ml quercetin, 0.45mg / ml baicalin, 0.020mg / ml ml forsythin reference substance solution;
[0081] B. Preparation of the test solution:
[0082] Precisely draw 25.0ml of compound Houttuynia cordata mixture (batch number: 130708), nine copies, and carry out the test: design the extraction solvent, solvent dosage and extraction times according to the L9(34) orthogonal table, and perform orthogonal according to Table 2, see the orthogonal results Table 3 to Table 6. Combine the extracts, evaporate to dryness, add 10ml of water to the residue to dissolve, pass through a D101 macroporous resin column (inner diameter 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com