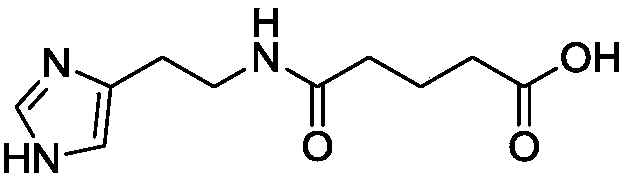
A kind of impurity detection method of ingaverine and preparation thereof
A detection method, the technology of ingavirin, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficult to evaluate the quality of ingavirin ingavirin capsules, less monitoring of important impurities, etc., to achieve accurate Quality control method, low cost, scientific detection method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
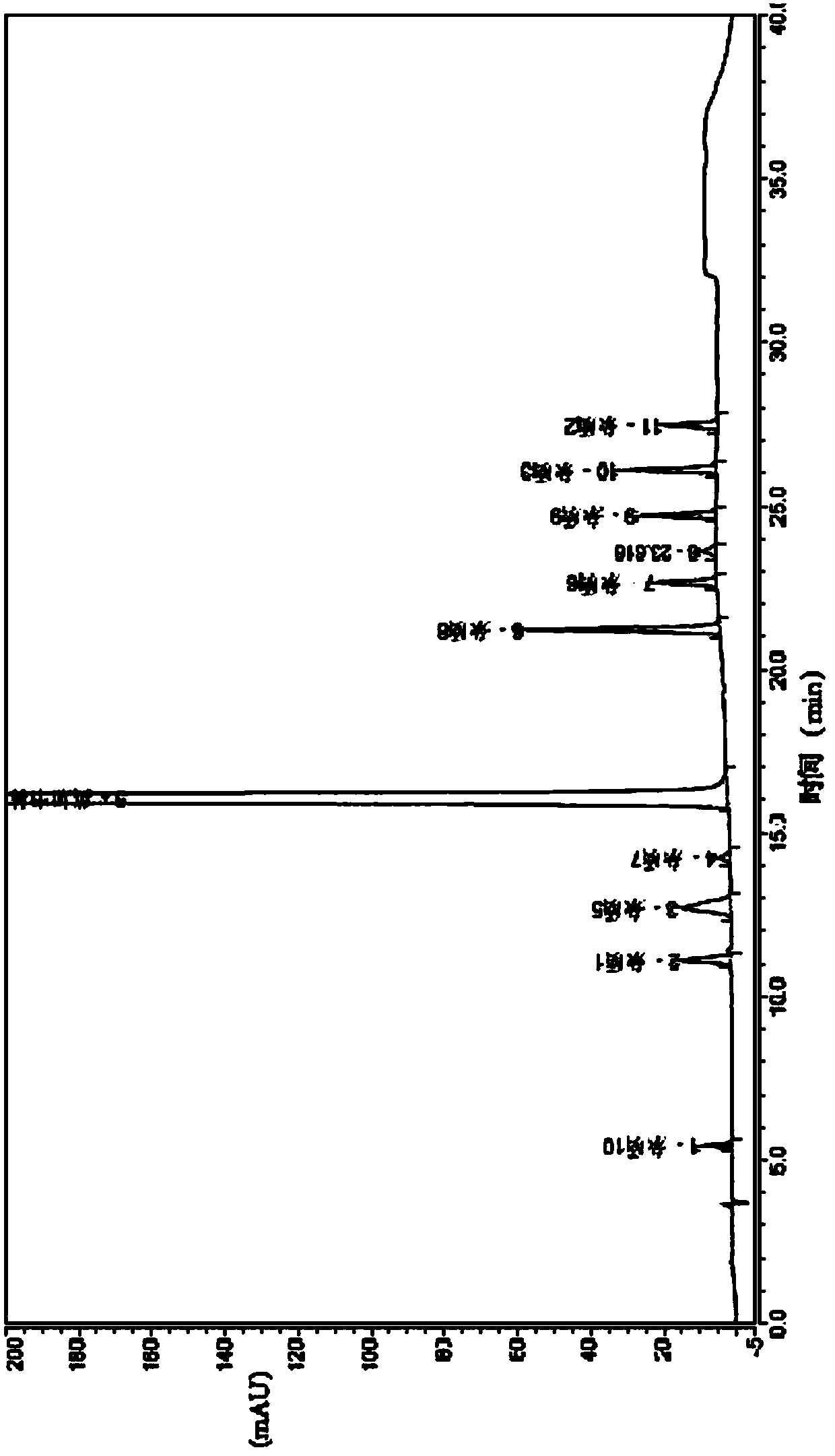
[0037] Embodiment 1 High Performance Liquid Chromatography Determination of Impurities in Ingaverine
[0038] Instrument and equipment samples:
[0039] Detector: UV detector, the detection wavelength is 214nm;
[0040] Chromatographic column: Octadecylsilane bonded silica gel (Thermo Syncronis C 18 , 4.6×250mm, 5μm applicable)
[0041] Column temperature: 35°C;
[0042] Mobile phase: the organic phase is a phosphate buffer at pH=2.6; the aqueous phase is methanol;
[0043] Mobile phase flow rate: 1.5ml / min
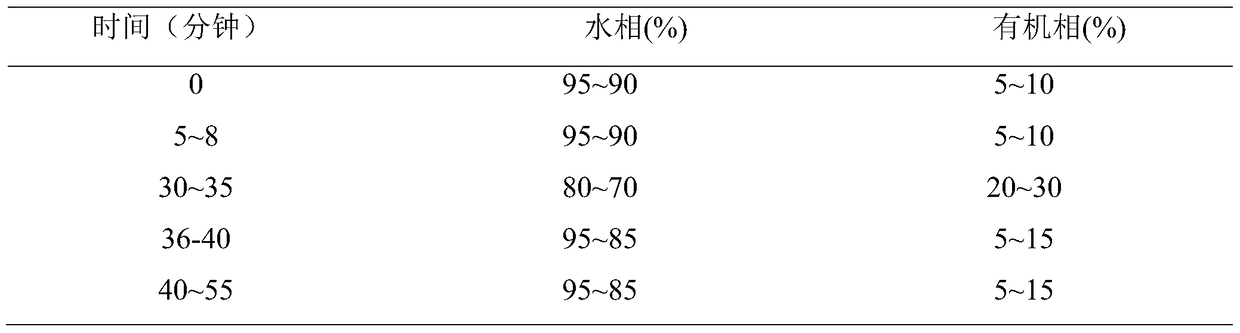
[0044] Carry out gradient elution according to the following table, elution conditions:
[0045]
[0046] Detection steps: take an appropriate amount of ingaverin, accurately weighed, add the initial mobile phase to dissolve and quantitatively dilute to make a solution containing about 1.0 mg of ingaverin per 1 ml, as the test solution; accurately pipette the test product Put 1ml of the solution in a 100ml measuring bottle, add the initial mobile phase and dilute...
Embodiment 2
[0050] Embodiment 2 Determination of impurities in ingaverine by high performance liquid chromatography
[0051] Instrument and equipment samples:
[0052] Detector: UV detector, the detection wavelength is 210nm;
[0053] Chromatographic column: Octadecylsilane bonded silica gel (Thermo Syncronis C 18 , 4.6×250mm, 5μm applicable)
[0054] Column temperature: 30°C;
[0055] Mobile phase: the organic phase is a phosphate buffer at pH=2.0; the aqueous phase is methanol;
[0056] Mobile phase flow rate: 1.5ml / min
[0057] Carry out gradient elution according to the following table, elution conditions:
[0058]
[0059] Assay steps are the same as in Example 1, and the test results are shown in Table 2.
[0060] The degree of separation between the impurities of table 2 embodiment 2
[0061] Peak order
Embodiment 3
[0062] Embodiment 3 High-performance liquid chromatography measures impurities in Ingaverine Capsules
[0063] Instrument and equipment samples:
[0064] Detector: UV detector, the detection wavelength is 214nm;
[0065] Chromatographic column: Octadecylsilane bonded silica gel (Thermo Syncronis C 18 , 4.6×250mm, 5μm applicable)
[0066] Column temperature: 50°C;
[0067] Mobile phase: the organic phase is a phosphate buffer at pH=3.0; the aqueous phase is methanol;
[0068] Mobile phase flow rate: 0.5ml / min
[0069] Carry out gradient elution according to the following table, elution conditions:
[0070]
[0071]Detection steps: Take an appropriate amount of the content of this product under the item of loading difference, add appropriate amount of water to dissolve ingaverin, and quantitatively dilute to make a solution containing about 1mg of ingaverin per 1ml, shake well, filter, and take Continue filtrate as need testing solution; Accurately pipette need testing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com