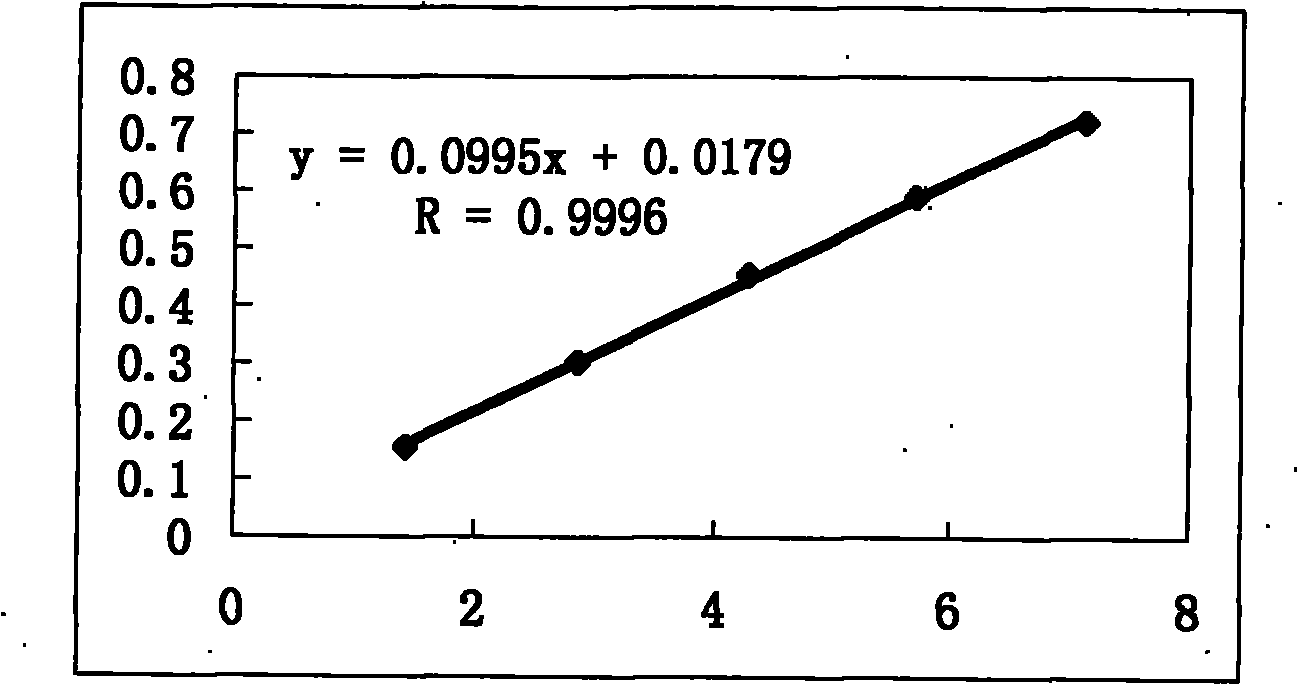Quality control method for pharmaceutic preparation containing sanguis draconis extract
A technology for pharmaceutical preparations and extracts, which is applied in the field of quality control of pharmaceutical preparations, can solve the problems of insufficient accuracy of thin-layer identification and spectrophotometry, and few detection items.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0287] Embodiment 1: the content determination method of total phenol:
[0288] a. Preparation of the reference substance solution: take an appropriate amount of 7,4′-dihydroxyflavone, accurately weigh it, put it in a brown measuring bottle, add absolute ethanol to make a solution containing 28 μg per 1 ml, and obtain it;
[0289] b. Preparation of standard curve: Accurately measure reference substance solution 0, 1.0, 2.0, 3.0, 4.0, 5.0ml, put them in 25ml measuring bottles respectively, add absolute ethanol to 5ml, add 0.3% dodecylsulfonic acid Sodium solution 2ml, 0.6% potassium ferricyanide solution 1ml, 0.9% ferric chloride solution 1ml, shake well, put it in a dark place for 5min, add 0.1M hydrochloric acid to the mark, shake well, put it away from light Place it for 35 minutes, and take the first copy as a blank; according to the UV-visible spectrophotometry method of Appendix VB of the Chinese Pharmacopoeia in 2005, measure the absorbance at 760nm, take the absorbance ...
Embodiment 2
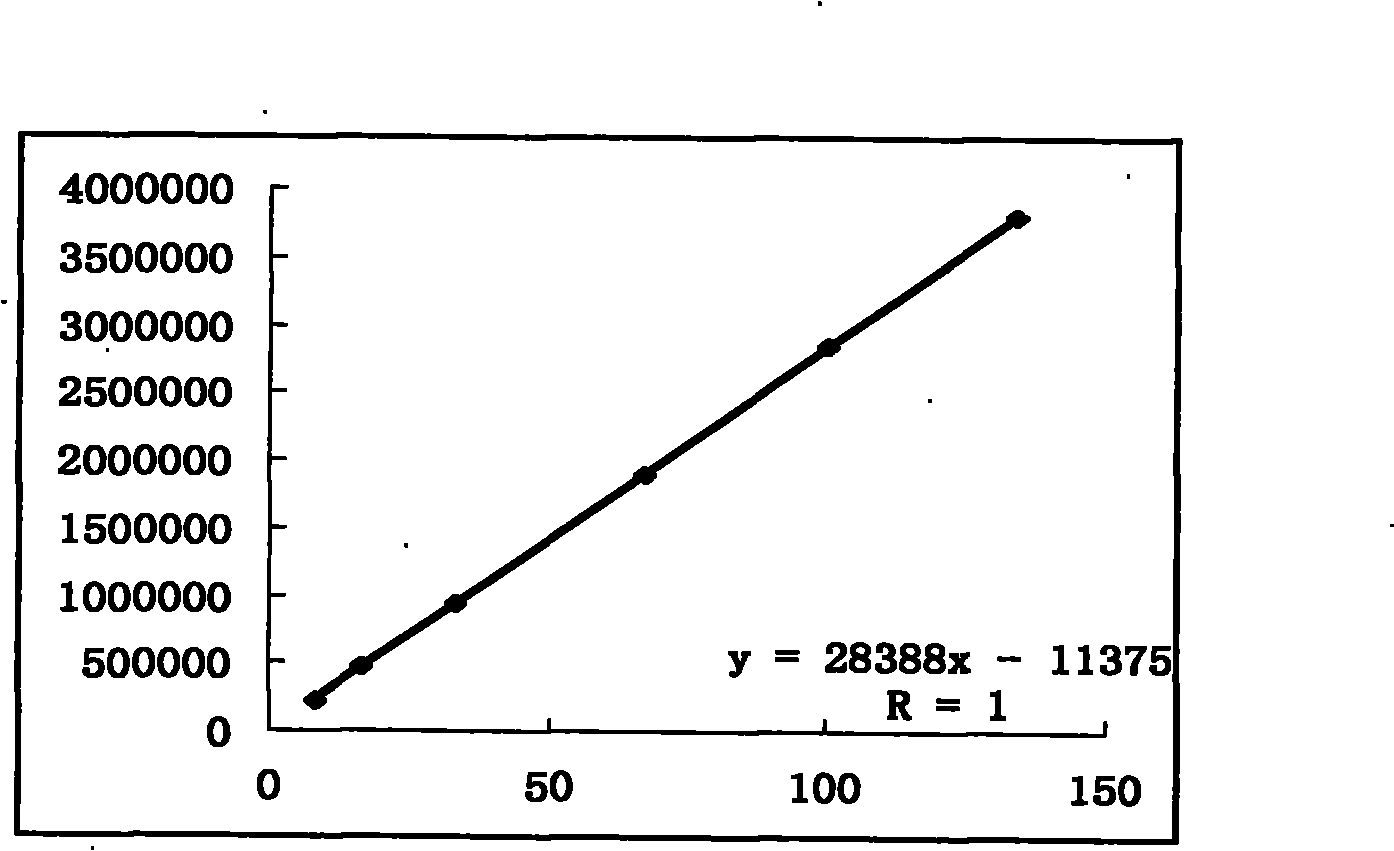
[0292] Embodiment 2: the content determination method of total phenols:
[0293] a. Preparation of the reference substance solution: take an appropriate amount of 7,4′-dihydroxyflavone, accurately weigh it, put it in a brown measuring bottle, add absolute ethanol to make a solution containing 32 μg per 1 ml, and obtain it;
[0294] b. Preparation of standard curve: Accurately measure reference substance solution 0, 1.0, 2.0, 3.0, 4.0, 5.0ml, put them in 25ml measuring bottles respectively, add absolute ethanol to 5ml, add 0.3% dodecylsulfonic acid Sodium solution 2ml, 0.6% potassium ferricyanide solution 1ml, 0.9% ferric chloride solution 1ml, shake well, put it in a dark place for 5min, add 0.1M hydrochloric acid to the mark, shake well, put it away from light place at 780nm according to the ultraviolet-visible spectrophotometry method of appendix VB of the Chinese Pharmacopoeia in 2005, measure the absorbance at 780nm, take the absorbance as the ordinate, and the concentrati...
Embodiment 3
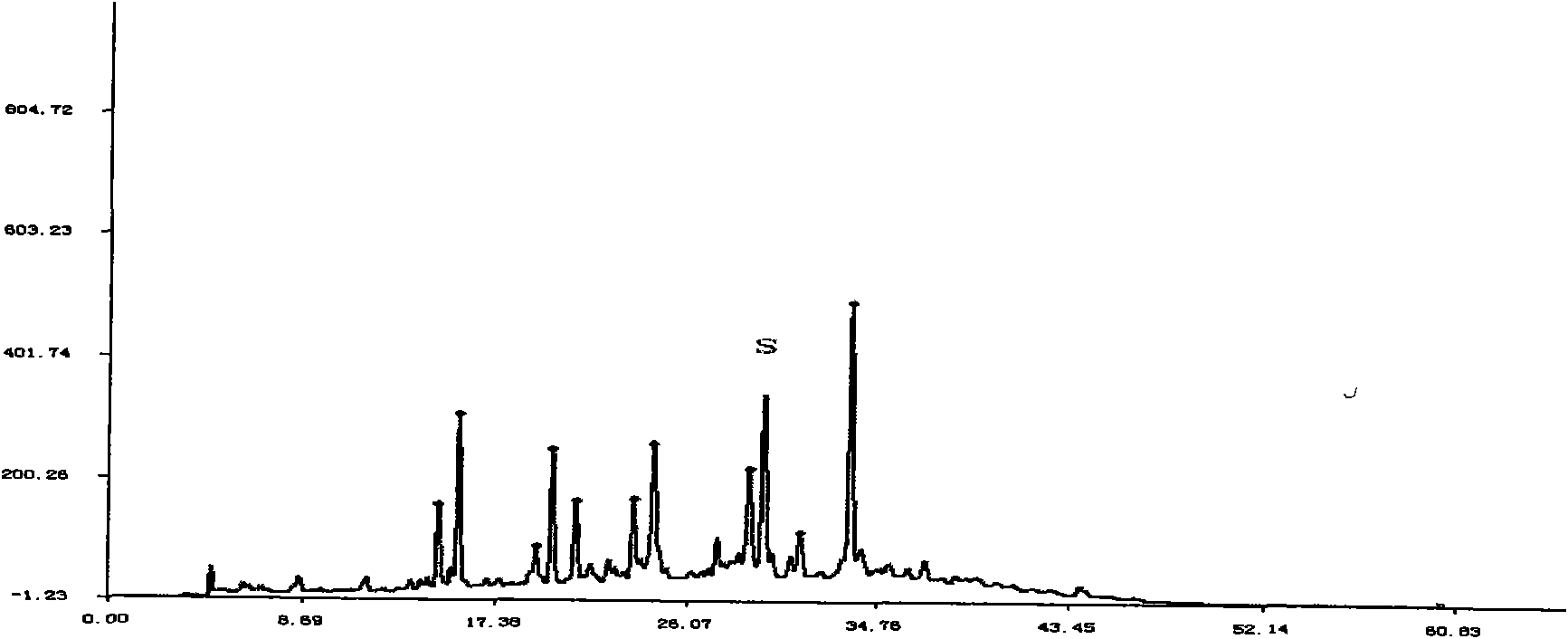
[0297] Embodiment 3: the content determination method of total phenol:
[0298] a. Preparation of the reference substance solution: take an appropriate amount of 7,4′-dihydroxyflavone, accurately weigh it, put it in a brown measuring bottle, add absolute ethanol to make a solution containing 36 μg per 1 ml, and obtain it;
[0299] b. Preparation of standard curve: Accurately measure reference substance solution 0, 1.0, 2.0, 3.0, 4.0, 5.0ml, put them in 25ml measuring bottles respectively, add absolute ethanol to 5ml, add 0.3% dodecylsulfonic acid Sodium solution 2ml, 0.6% potassium ferricyanide solution 1ml, 0.9% ferric chloride solution 1ml, shake well, put it in a dark place for 5min, add 0.1M hydrochloric acid to the mark, shake well, put it away from light Place it for 35 minutes, and take the first copy as a blank; according to the ultraviolet-visible spectrophotometry method of appendix VB of the Chinese Pharmacopoeia in 2005, measure the absorbance at 800nm, take the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com