Method for detecting liver-enhancing medicine
A detection method and drug technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as relatively difficult and complex interactions of traditional Chinese medicine components, and achieve the effects of easy mastery, perfect quality control methods, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
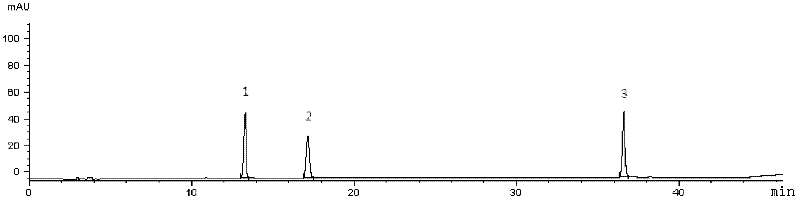
[0072] Embodiment 1 adopts HPLC to establish the multi-component assay method of Qianggan capsule content
[0073] Preparation of mixed reference solution: Accurately weigh gentiopicroside 7.55mg, paeoniflorin 3.76mg, and salvianolic acid B 5.66mg respectively into 25ml measuring bottles, dissolve and dilute with methanol to the mark, shake well, and use as stock solutions. Precisely measure 2ml of gentiopicroside, paeoniflorin stock solution, and salvianolic acid B stock solution into a 10ml measuring bottle, dilute to the mark with methanol, and shake well to obtain a mixed reference solution.
[0074] Preparation of the test solution: Weigh about 0.3g of the content of Qianggan Capsules, accurately weigh, place in a conical flask or a 25ml volumetric flask, accurately add 25mL of 70% volume fraction methanol aqueous solution, weigh, sonicate or heat to reflux After 30 minutes, put it to room temperature, make up the weight with 70% methanol aqueous solution, shake well, f...
Embodiment 2
[0083] The investigation of need testing solution preparation method in the multi-component detection method of embodiment 2
[0084] Investigation on the preparation method of the test solution
[0085] 1. Investigation of extraction solvent
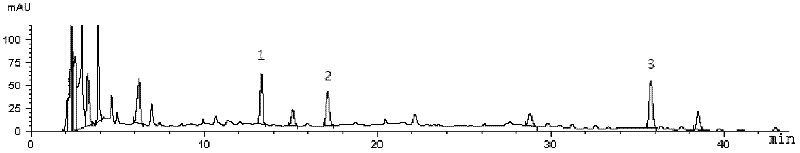
[0086] Take about 0.5g of the sample, weigh it accurately, add methanol, 70% methanol water solution, 75% ethanol water solution, and 50% ethanol water solution each 25ml as the extraction solvent, seal it tightly, weigh it, ultrasonicate for 30 minutes, take it out, and place it until At room temperature, weigh, make up the weight with the corresponding solvent, shake well, filter through a 0.45 μm microporous membrane, and take the subsequent filtrate for determination according to the determined chromatographic conditions. The test data are shown in Table 3 below.
[0087] The selection test data of table 3 extraction solvent
[0088] solvent for extraction
Gentiopicroside (area / g)
Paeoniflorin (area / g)
Salv...
Embodiment 3
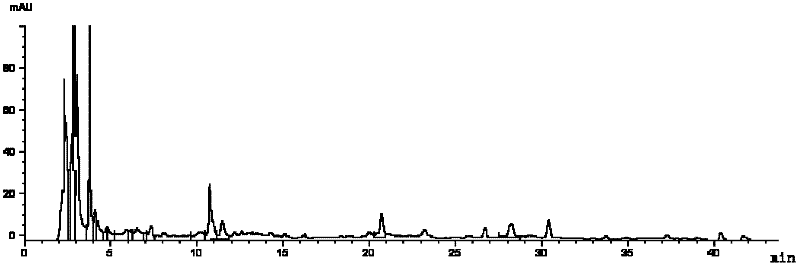
[0105] Methodological investigation of embodiment 3 multi-component detection method
[0106] 1) Precision test
[0107] Adopt the operation and condition identical with embodiment 1, get about 0.3g of sample, accurately weigh, process by need testing solution preparation method, precision draw need testing solution 10 μ L, inject high-performance liquid chromatograph, in above-mentioned chromatographic condition The RSD values were 0.55%, 0.69% and 1.48% for 6 consecutive injections. The precision is good.
[0108] 2) Stability inspection
[0109] Using the same operation and conditions as in Example 1, take about 0.3 g of the sample, prepare and extract according to the test solution, inject samples at 0, 1, 3, 6, 9, 12, and 24 hours respectively, and measure under the above-mentioned chromatographic conditions . As a result, the RSD of gentiopicroside was 0.77%, the RSD of paeoniflorin was 1.09%, and the RSD of salvianolic acid B was 0.75%. It shows that the test s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com