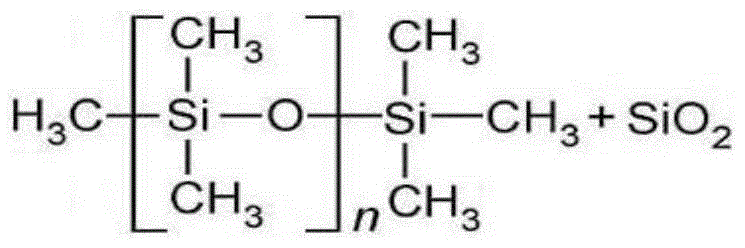
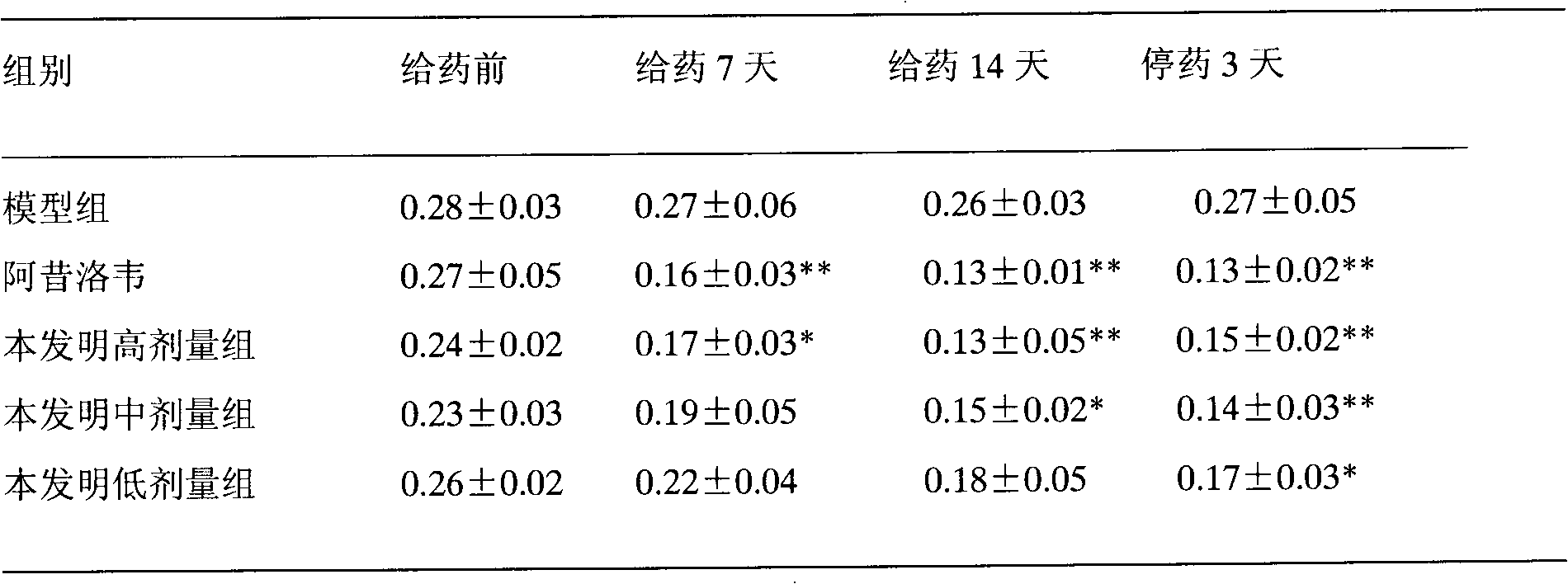
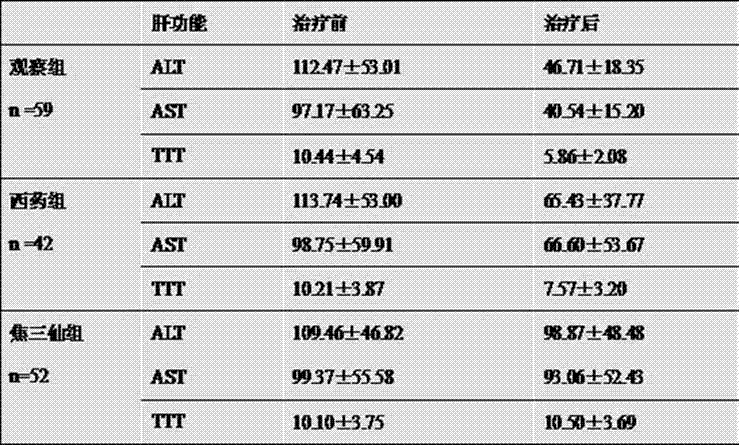
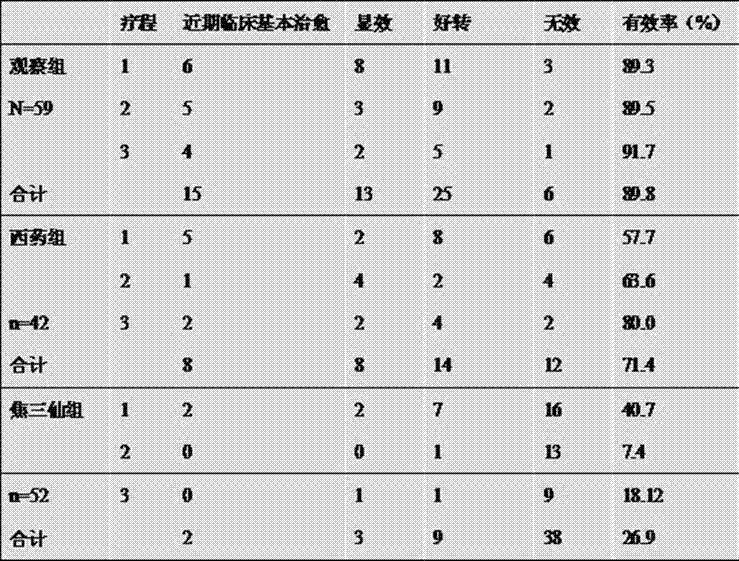
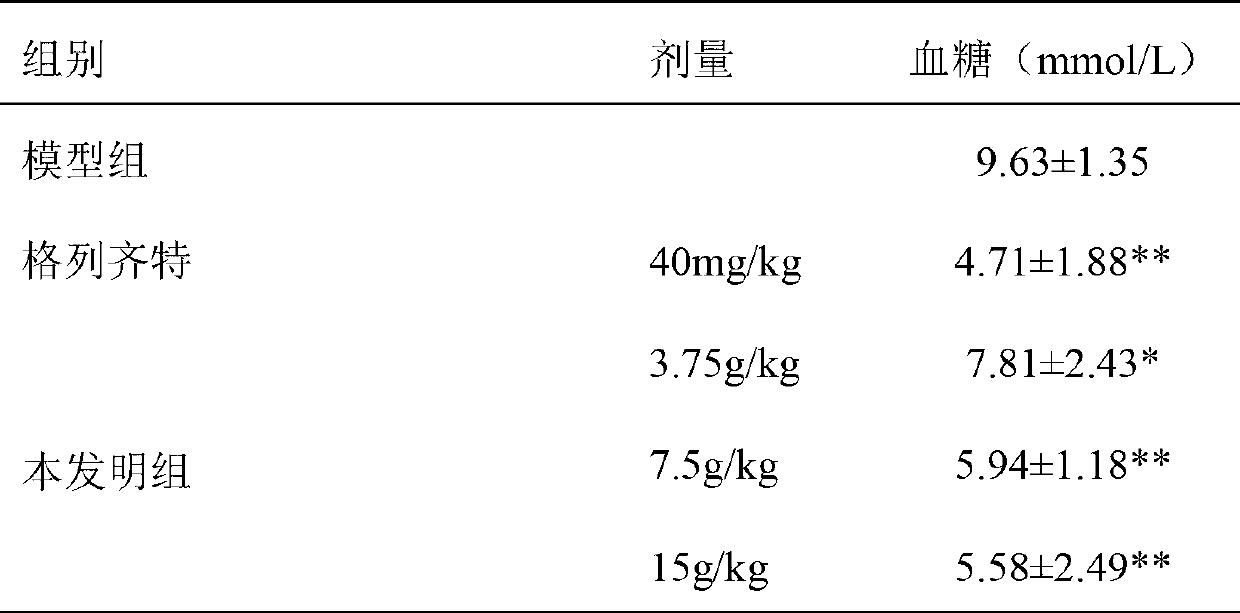
Patents
Literature
167results about How to "Reasonable prescription design" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium-potassium citrate chewing tablet and preparation method thereof
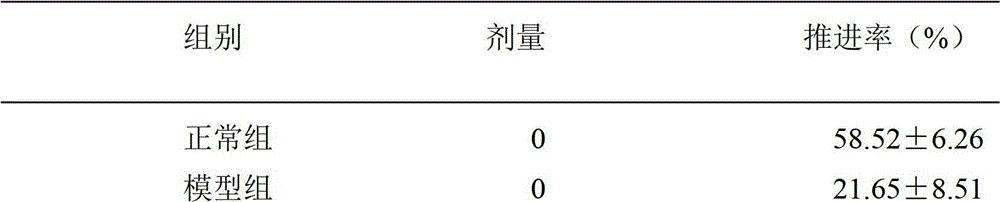
ActiveCN102429887AStable and controllable qualityCool tasteOrganic active ingredientsMetabolism disorderCelluloseMagnesium stearate
The invention provide a sodium-potassium citrate chewing tablet, of which the prescription is composed of the following components by mass percent: 33.0-33.1% of potassium citrate, 27.8-27.9% of sodium citrate, 28-32% of filling agent, 0.05-0.3% of adhesive, 1-1.5% of lubricant, 3-5% of flavoring agent, 0.1-0.3% of aromatizer and 3-4% of moistureproof film coating agent, wherein the filling agentis mannitol or a mixture of mannitol and sorbitol or xylitol; the adhesive is hydroxypropyl methylcellulose or polyvidone K30; the lubricant is magnesium stearate or a mixture of magnesium stearate and micropowder silica gel; the flavoring agent is citric acid or a mixture of citric acid and sodium saccharin, aspartame or steviosin; and the aromatizer is pharmaceutically acceptable essence. The invention also provides a preparation method of the sodium-potassium citrate chewing tablet. The preparation method is simple and convenient to operate, low in cost and suitable for industrial production. The obtained tablet has stable and controllable quality, fresh and cool mouthfeel, sourness and sweetness in taste, mint fragrance or fruit fragrance, smooth and beautiful surface, uniform color, moderate hardness and rapid dissolution, and has good application prospects in treatment of gout and hyperuricemia as well as improvement of children and adult in vivo acidosis symptom and other aspects.
Owner:SOUTHWEST UNIV
Chinese medicinal composition for treating disorders of digestive function and preparation method and application thereof
ActiveCN102302728AReasonable designStrict compatibilityDigestive systemMammal material medical ingredientsAdemetionineRice grain
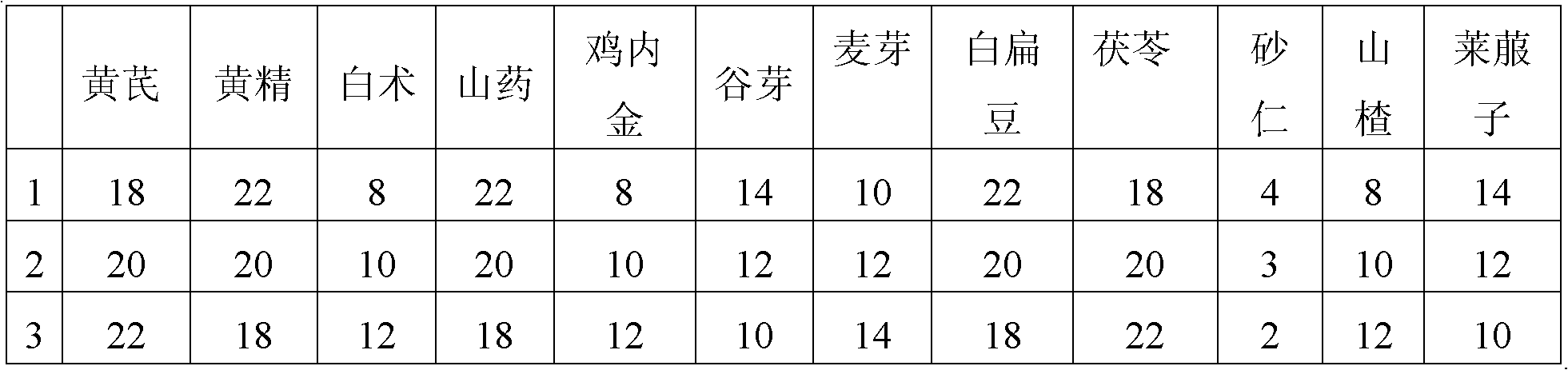
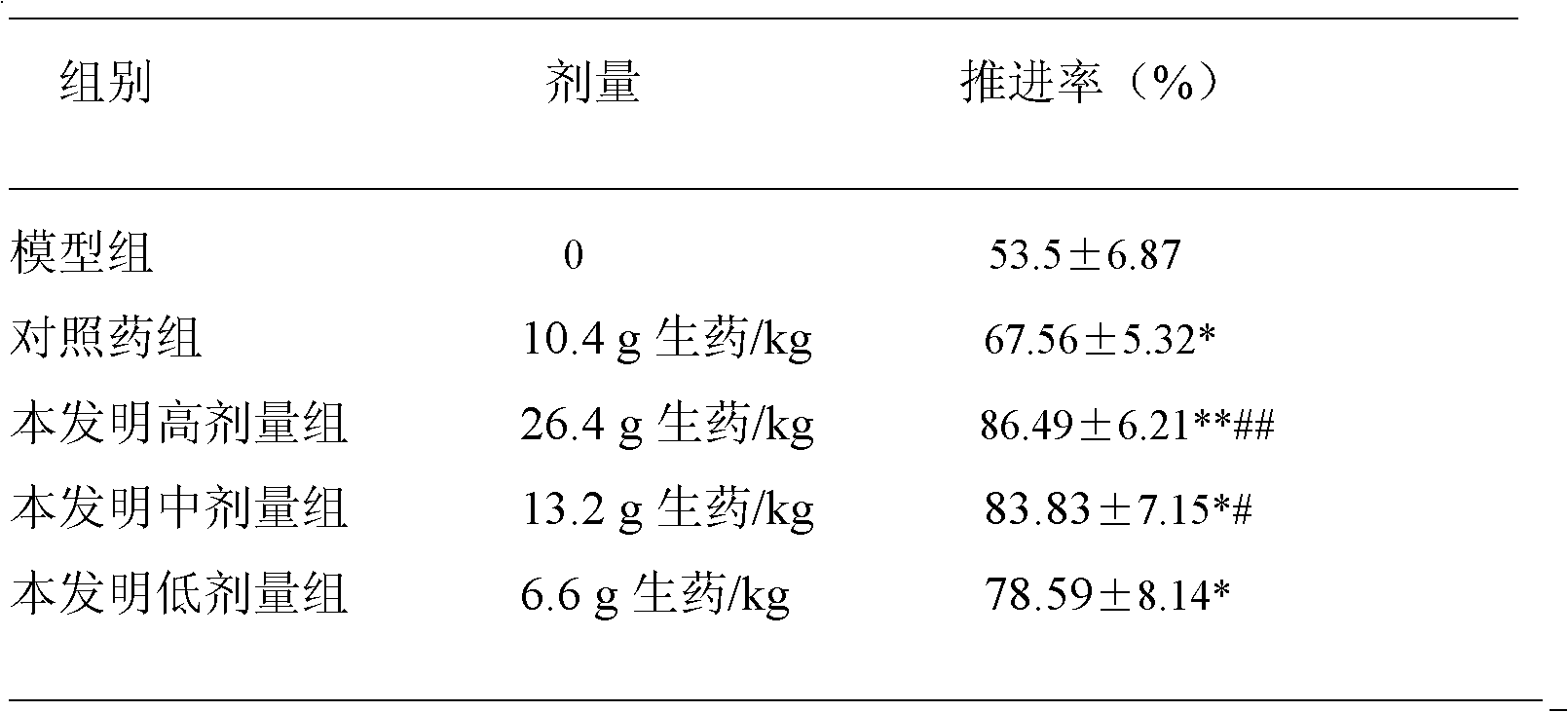
The invention provides a Chinese medicinal composition for treating disorders of digestive function and a preparation method and application thereof. The Chinese medicinal composition is mainly prepared from the following Chinese herbal medicines in part by weight: 18 to 22 parts of astragalus, 18 to 22 parts of manyflower solomonseal rhizome, 8 to 12 parts of white atractylodes rhizome, 18 to 22parts of yam, 8 to 12 parts of membrane, 10 to 14 parts of rice grain sprout, 10 to 14 parts of malt, 18 to 22 parts of white hyacinth bean, 18 to 22 parts of tuckahoe, 2 to 4 parts of amomum fruit, 8 to 12 parts of hawthorn and 10 to 14 parts of radish seed, and can be prepared into oral liquid, tablets, capsules or granules by the corresponding preparation method. The Chinese medicinal composition has the functions of invigorating qi and strengthening the spleen and is used for treating disorders of digestive function.
Owner:南通金羽禽业发展有限公司
Ibuprofen diphenhydramine orally disintegrating tablet and preparation method thereof
ActiveCN102488681AReasonable prescription designSimple and efficient operationOrganic active ingredientsNervous disorderSodium carboxymethylcelluloseActive agent
The invention provides an ibuprofen diphenhydramine orally disintegrating tablet, comprising the following ingredients, by weight: 40% of ibuprofen, 5% of diphenhydramine hydrochloride or 7.6% of diphenhydramine citrate, 35-40% of filler, 6-12% of disintegrating agent, 0-5% of effervescent disintegrant, 1-2% of adhesive, 1-2.5% of surfactant, 1-1.5% of lubricant, 0.7-2.5% of sweetener and 0-0.5% of perfume. The filler is at least two from microcrystalline cellulose, mannitol and pregelatinized starch; the disintegrating agent is at least one from crospovidone, cross linked sodium carboxymethyl cellulose and sodium carboxymethyl starch; the effervescent disintegrant comprises citric acid and sodium bicarbonate in a mass ratio of 1-2:1; the adhesive is hydroxypropyl methylcellulose; the surfactant is sodium dodecyl sulfate; the lubricant is eleaostearic acid or magnesium stearate and colloid silica; the sweetener is aspartame and / or steviosin; and the perfume is medicinal essence. Theinvention also provides a preparation method of the orally disintegrating tablet and is simply operated, at low cost and suitable for industrialized production; and the obtained product satisfies quality requirement of an orally disintegrating tablet and has beautiful appearance, good mouthfeel and stable quality.
Owner:SOUTHWEST UNIVERSITY
Traditional Chinese medicine composition for treating hyperlipemia, and preparation method and application thereof
ActiveCN102228577AEnhanced digestive stagnation effectReasonable prescription designMetabolism disorderPlant ingredientsSalvia miltiorrhizaMedicine
The invention provides a traditional Chinese medicine composition for achieving the purpose of treating hyperlipemia through promoting blood circulation, removing blood stasis, eliminating dampness and removing stagnation. To achieve the purpose, the invention adopts the following technical scheme that: the active components contained in the traditional Chinese medicine for treating hyperlipemia are prepared from the following raw materials in parts by weight: 10-30 parts of salvia miltiorrhiza, 5-15 parts of hawthorn, 5-15 parts of cherokee rose fruit, 5-20 parts of oriental waterplantain rhizome, 10-30 parts of fleece-flower root and 10-30 parts of radish seed, and a corresponding preparation method is adopted. The traditional Chinese medicine composition has the efficacies of promoting blood circulation, removing blood stasis, eliminating dampness, removing stagnation and treating hyperlipemia.
Owner:HUNAN SAILONG PHARMA
A deer bone medicinal wine for treating fractures
InactiveCN102283917AReasonable prescription designStrict compatibilityHeavy metal active ingredientsSkeletal disorderWhite liquorMedicine
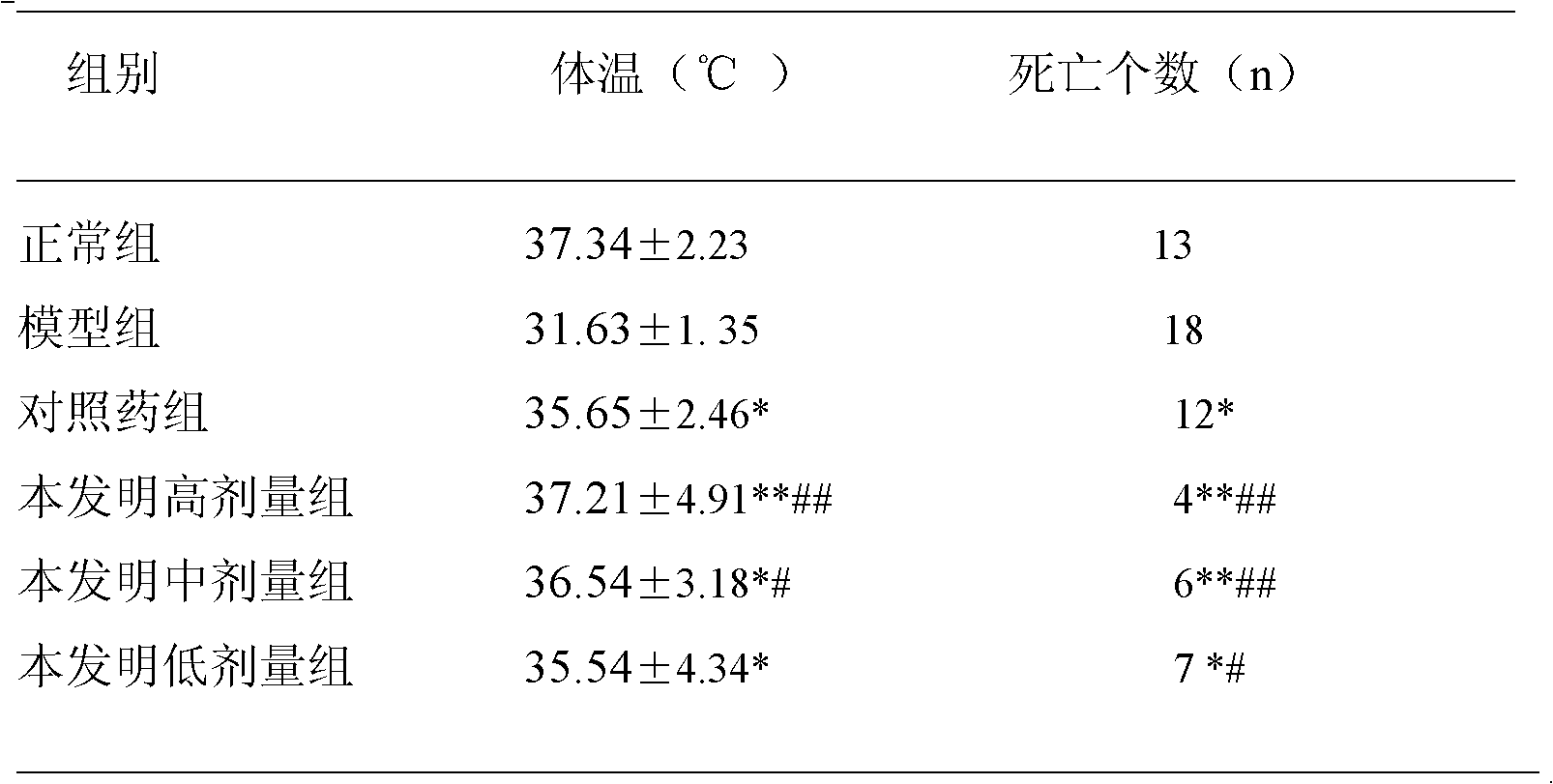
The invention provides a deer bone medicated liquor for treating fracture, which is prepared by carrying out impurity removal and crushing deer bone, lignum sappan, peach seed, safflower, redroot gromwell, radix paeoniae rubra, rhizome chuangxiong, net cliffbean, pyritum and combined spicebush root medicinal materials, passing through a sieve with 60 meshes, feeding the mixture into white liquor with 30-50DEG C and soaking for 10-20 days; filtering decoction dredges to obtain the medicated liquor. The medicated liquor achieves the aims of treating the fracture by strengthening the muscles andbones, expelling wind and removing dampness, promoting blood circulation by removing blood stasis and eliminating stagnation to stop pain, can be used for quickly and effectively treating the fracture and meeting the requirements of mostly fracture patients; and the deer bone medicated liquor has the advantages of low possibility of relapse, simpleness in formula, low cost and easiness in production.
Owner:淮安市淮安区古神梅花鹿养殖场
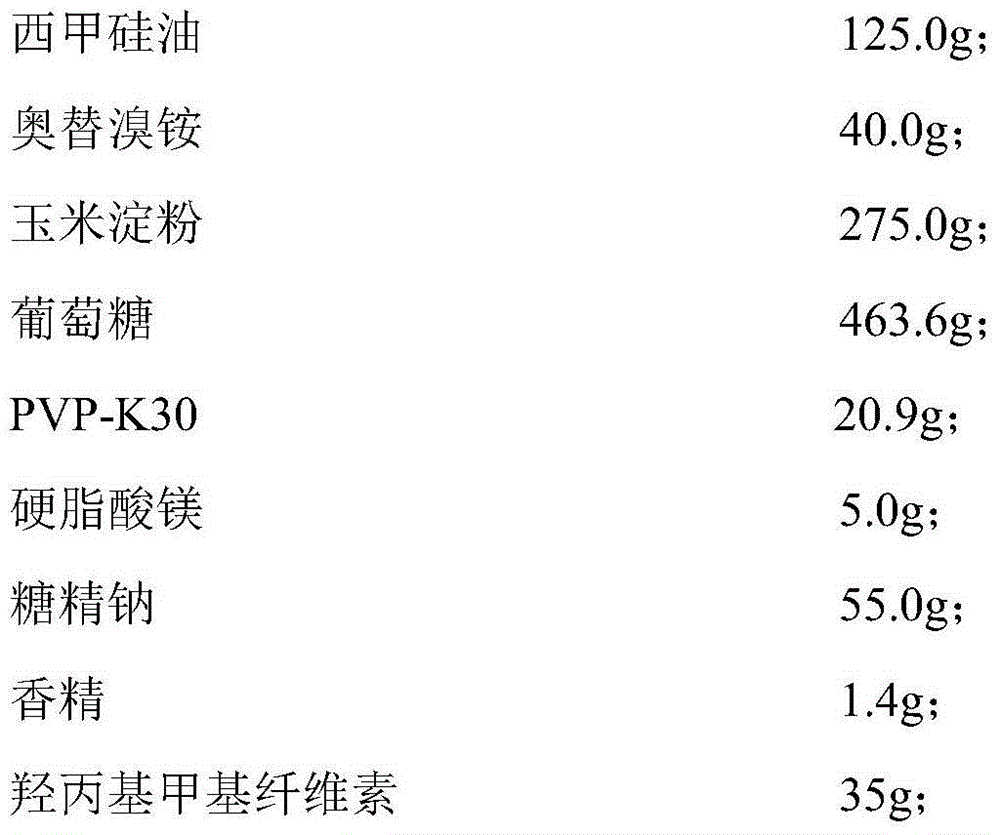
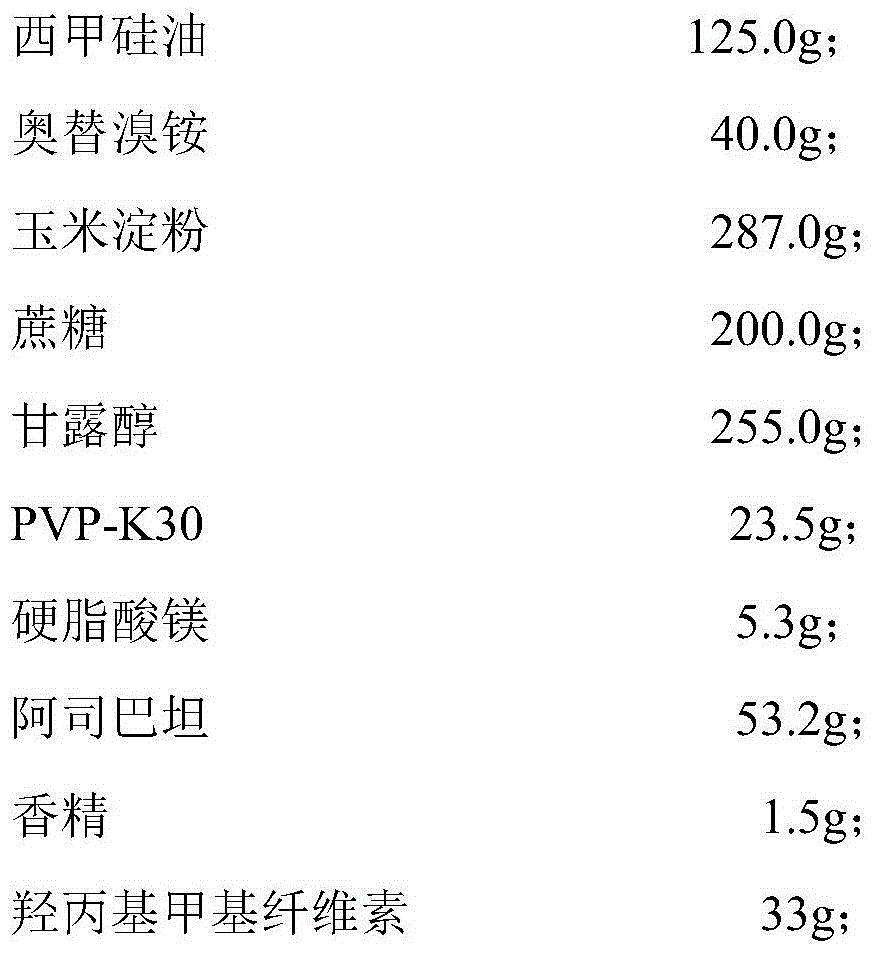
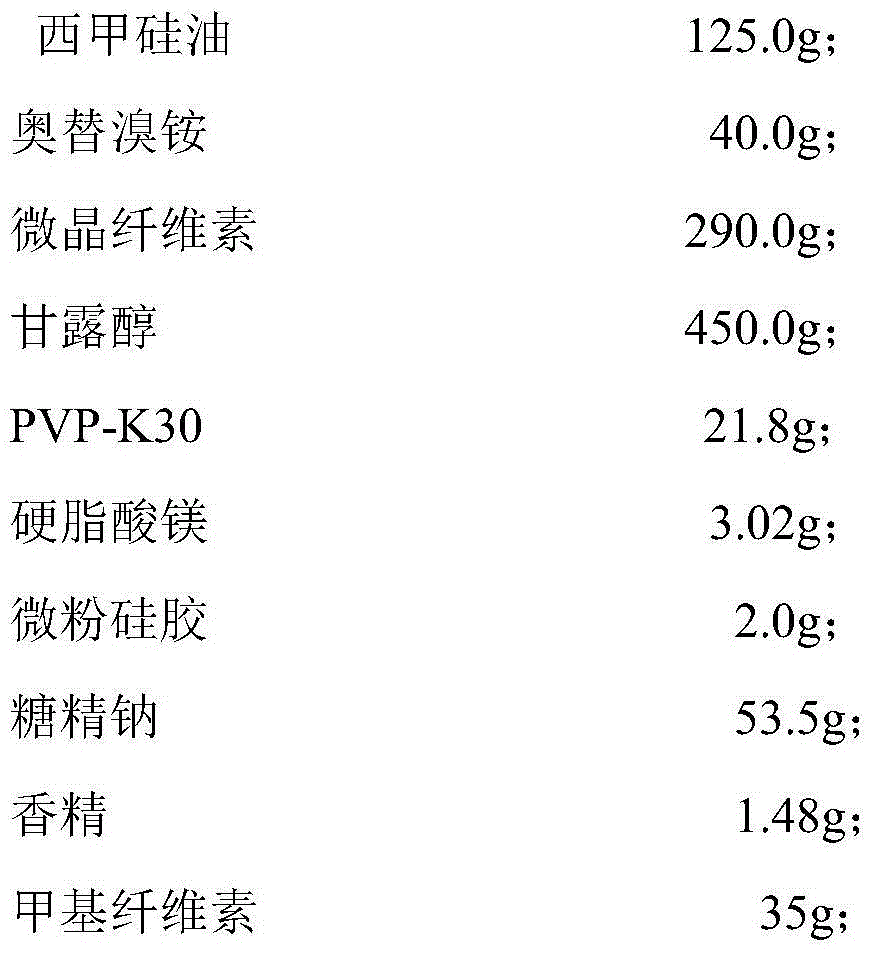
Simethicone dry suspension and preparation method thereof
ActiveCN105055326AQuality improvementGreat tasteDigestive systemSilicon compound active ingredientsDiseaseEmulsion
The invention discloses a simethicone dry suspension and a preparation method thereof. The simethicone dry suspension contains simethicone of 4% in mass percentage and a carrier which is pharmaceutically acceptable. The preparation method is simple and convenient in operation, low in cost and suitable for industrialized production. The simethicone dry suspension has the advantages of good taste, uniform distribution, high dissolubility and stable quality, is more advantageous and convenient than conventional liquid simethicone suspensions and emulsions in aspects of packaging, carrying and transporting, is used for treating diseases like abdominal distension, abdominal discomfort, indigestion and postoperative abdominal distension caused by abdominal gas gathering and has good application prospect.
Owner:SOUTHWEST UNIVERSITY
Drug composition for treating acute pharyngitis
InactiveCN103585336AReasonable designStrict compatibilityInorganic boron active ingredientsHydroxy compound active ingredientsToxinCypress
The invention provides a drug composition for treating acute pharyngitis. The drug composition is prepared from the following raw materials in parts by weight: 0.5-1.5 parts of burdock, 8-12 parts of borax, 8-12 parts of platycodon grandiflorum, 8-12 parts of indigo naturalis, 2-4 parts of wild chrysanthemum flowers, 4-8 parts of mint, 4-6 parts of golden cypress, 2-4 parts of liquorice and 2-4 parts of borneol. The drug composition achieves the purpose of treating the acute pharyngitis by easing pain, diminishing inflammation, clearing heat and toxins, eliminating stagnation and relieving sore-throat, and is easy to prepare and convenient to take.
Owner:钱家美
Medicine for treating depression, and preparation method and application thereof
InactiveCN102416148AIncrease weightReduce consumptionHeavy metal active ingredientsNervous disorderOysterAcorus gramineus
The invention discloses a medicine for treating depression, and a preparation method thereof. The medicine is prepared from bupleurum chinense, root of red-rooted salvia, acorus gramineus, thinleaf milkwort rootbark, zedoary, coptis chinensis, dragon bone, oyster, and chlorite schist in a certain weight part proportion; bupleurum chinense, root of red-rooted salvia, acorus gramineus, thinleaf milkwort rootbark, zedoary and coptis chinensis are extracted by a percolation method, dragon bone, oyster, and chlorite schist are extracted by a levigation method, then extracts are mixed, and mixed extracts and auxiliary materials are mixed to be prepared into any common oral formulation. The medicine has the effects of soothing the liver and regulating vital energy, activating blood and dissolving stasis, tranquilizing the mind, reducing phlegm and purging fire and the like, is used for treating liver qi stagnation, vexation and sleeplessness and other symptoms, and is also used for treating depression.
Owner:NANJING HAIYUAN CHINESE HERBAL PIECES CO LTD
Medicinal liquor for treating fracture as well as preparation method and application of medicinal liquor
InactiveCN103989744APromote osteosynthesisReasonable prescription designAntipyreticAnalgesicsRemove bloodEuchresta japonica
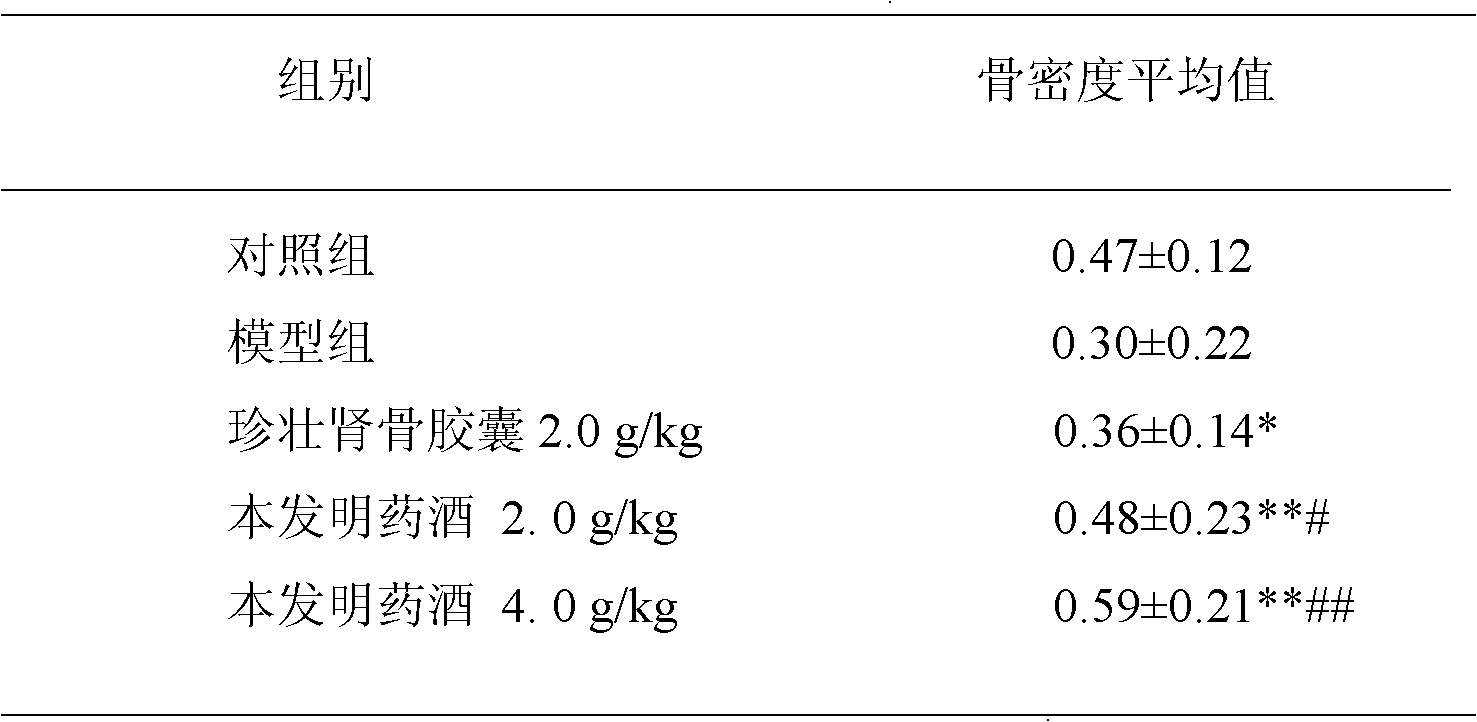
The invention provides medicinal liquor for treating fracture. The medicinal liquor is prepared from the following raw materials in parts by weight: 20-30 parts of obovateleaf actinodaphne bark, 15-25 parts of Euchresta japonica, 8-12 parts of all-grass of Tali Lobelia, 8-12 parts of lanceleaf magnoliavine herb, 10-14 parts of rhizoma drynariae and 18-22 parts of the root of three-nerved spicebush. The aim of treating fracture is achieved through the aims of strengthening tendons and bones, dispelling wind and eliminating dampness, promoting blood circulation to remove blood stasis and eliminating stagnation stop pain. The invention also discloses a preparation method and application of the medicinal liquor.
Owner:徐艳
Traditional Chinese medicine combination for treating hepatitis B and preparing method and application thereof
InactiveCN102626468AReasonable prescription designStrict compatibilityDigestive systemAntiviralsPatriniaToxic material
The invention provides a traditional Chinese medicine combination, which achieves the aim of treating hepatitis B by clearing away heat and toxic materials and eliminating dampness and phlegm. For achieving the aim, the technical scheme of the traditional Chinese medicine is that active ingredients contained by the combination are prepared by the following raw materials by weight: 20-40 parts of oriental wormwood, 40-60 parts of desmodium, 10-30 parts of patrinia, 5-15 parts of Polygonum cuspidatum, 20-40 parts of Oldenlandia diffusa, 5-15 parts of herb of snow of june, 5-15 parts of Rhizoma Atractylodis Macrocephalae, 10-30 parts of the fruit of Chinese wolfberry, 20-40 parts of fructus ligustri lucidi, 20-40 parts of hawthorn and 5-15 parts of liquorice. By adopting the corresponding preparing method, the traditional Chinese medicine combination has the advantages of clearing away heat and toxic materials, eliminating dampness and phlegm and treating hepatitis B.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE +1
Traditional Chinese medicine composition for treating chronic virulent hepatitis b and preparation method thereof
InactiveCN104707108AGood anti-hepatitis B activityReasonable prescription designDigestive systemAntiviralsSalvia miltiorrhizaSide effect
The invention discloses a traditional Chinese medicine composition for treating chronic virulent hepatitis b and a preparation method thereof, and belongs to the technical field of Chinese patent medicine for treating hepatitis b. According to the technical scheme, the traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 55-72 parts of astragalus membranaceus, 58-75 parts of salvia miltiorrhiza, 40-56 parts of glossy privet fruit, 40-58 parts of Chinese wolfberry, 40-58 parts of rhizoma smilacis glabrae, 30-52 parts of radix bupleuri, 32-48 parts of poria cocos, 30-52 parts of schisandra chinensis, 30-58 parts of codonopsis pilosula, 28-56 parts of pinellia ternate, 28-58 parts of Chinese yam, 30-46 parts of isatis roots, 32-48 parts of bighead atractylodes rhizome, 30-46 parts of curcuma aromatica, 32-46 parts of radix paeoniae alba, 30-49 parts of oldenlandia diffusa, 32-49 parts of angelica sinensis, 35-46 parts of pericarpium citri reticulatae, 35-50 parts of honeysuckle, 30-44 parts of polygonatum sibiricum and 25-35 parts of coke hawthorn. The invention further discloses the preparation method for the traditional Chinese medicine composition for treating chronic virulent hepatitis b. The traditional Chinese medicine composition is prepared from traditional Chinese medicinal materials, has no toxic or side effect, and is low in cost. Animal experiments and clinical application studies find that the traditional Chinese medicine composition has good anti-HBV activity.
Owner:杨道坤
Traditional Chinese medicine composition for treating diabetes as well as preparation method and application of for traditional Chinese medicine composition
InactiveCN102961596AReasonable designStrict compatibilityMetabolism disorderPlant ingredientsAdemetioninePericarpium citri reticulatae
The invention provides a traditional Chinese medicine composition for treating diabetes. The traditional Chinese medicine composition is prepared from the following active pharmaceutical ingredients in parts by weight: 10-20 parts of leguminosae, 5-15 parts of rhizoma cimicifugae, 10-20 parts of rhizoma atractylodis macrocephalae, 15-25 parts of radix ophiopogonis, 15-25 parts of polygonatum sibiricum, 10-20 parts of spina date seed, 10-20 parts of radix polygalae, 10-20 parts of fructus corni, 10-20 parts of dioscorea opposita, 15-25 parts of fructus crataegi, 15-25 parts of pilose asiabell, 15-25 parts of pericarpium citri reticulatae, 30-50 parts of tuber fleeceflower stem, 15-25 parts of coptis chinensis franch, 10-20 parts of semen dolichoris album, 10-20 parts of processed fallopia multiflora and 10-20 parts of radix glycyrrhizae. The traditional Chinese medicine composition tonifies qi and blood, nourishes yin for lowering fire and regulates vital energy and swift digests to realize the purpose of treating diabetes. The invention further discloses a preparation method and application of the traditional Chinese medicine composition.
Owner:杨忠彬
Traditional Chinese medicine composition for treating constipation, preparation method and application
ActiveCN102940718AReasonable designStrict compatibilityDigestive systemPlant ingredientsPaeonia lactifloraAgeratum
The invention provides a traditional Chinese medicine composition for treating constipation, a preparation method and an application. The traditional Chinese medicine composition is mainly composed of the following components in parts by weight:18-22 parts of ageratum, 55-65 parts of cortex magnoliae officinalis, 18-22 parts of paeonia lactiflora pallas, 8-12 parts of delavay soapberry pericarp seed, 8-12 parts of dried old orange peel, 13-17 parts of almond, 10-14 parts of semen trichosanthis, 10-14 parts of angelica and 18-22 parts of peach kernels, and the composition is prepared to oral liquid, tablets, capsules or granules by a corresponding preparation method. The traditional Chinese medicine composition has the functions of tonifying middle-Jiao and Qi and invigorating and activating spleen, and the traditional Chinese medicine composition is used for treating constipation.
Owner:海门江海建设投资有限公司
Medicinal liquor for treating rheumatoid arthritis
ActiveCN102225102AImprove the effect of expelling wind and dredging collateralsReasonable prescription designAnthropod material medical ingredientsAntipyreticArthritisGLYCYRRHIZA EXTRACT
The invention provides a medicinal liquor for treating rheumatoid arthritis, which is mainly prepared by following steps: removing impurities in Chinese herbal medicines including radix stephaniae tetrandrae, radix clematidis, scorpion, zaocys dhumnades, radix astragali, angelica, ground beetle, liquorice, and the like, cleaning, soaking in a liquor of 40-60 degrees for 10-20 days, and filtering. The medicinal liquor for treating rheumatoid arthritis has fast effect, is not easy to recur, has simple formula, low cost, is easy to produce, and is suitable for demands of most rheumatoid arthritis patients.
Owner:南通康威尔生物化工有限公司
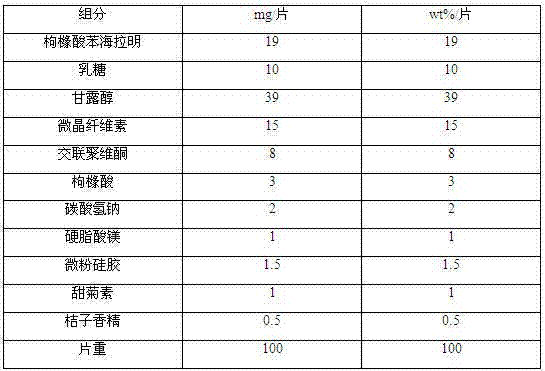
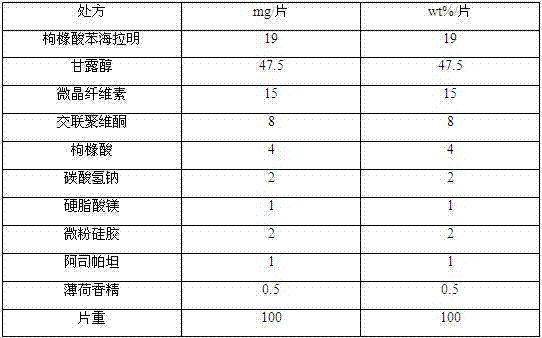
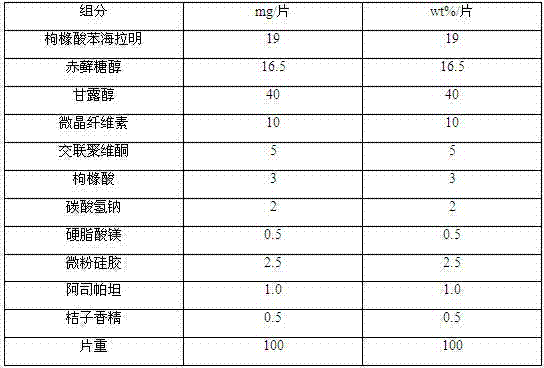
Diphenhydramine citrate orally disintegrating tablet and preparation method thereof
InactiveCN102440973AMeet quality requirementsReasonable prescription designOrganic active ingredientsNervous disorderSodium bicarbonateOrally disintegrating tablet
The invention discloses a diphenhydramine citrate orally disintegrating tablet, the prescription is composed by the following components in mass percent: 19% of diphenhydramine citrate, 45 swung dash 58% of filler, 15 swung dash 25% of disintegrating agent, 4 swung dash 8% of effervescent disintegrant, 0.5 swung dash 1% of lubricant, 1 swung dash 3% glidant, 1 swung dash 2% of sweetner, 0.2swung dash 0.6% aromatic and 0 swung dash 0.5% of surfactant, wherein the filler uses mannitol, or mannitol and lactose or erythritol, the disintegrating agent uses any one of polyplasdone, crosslinking sodium carboxy methyl cellulose and low substitution hydroxyl propyl cellulose together with microcrystalline cellulose, the effervescent disintegrant comprises citric acid and sodium bicarbonate which have the mass ratio of 1 swung dash 2 / 1, the lubricant is magnesium stearate, the glidant is aerosil or talcum powder, the sweetner is aspartame or stevia rebaudianum, the aromatic is the pharmaceutically acceptable essence, and the surfactant is lauryl sodium sulfate; and the invention further discloses a preparation method of the orally disintegrating tablet, which is simple to operate, the cost is low, the obtained product is in accordance with the quality requirement of the orally disintegrating tablet, and has attractive appearance, good taste and stable quality.
Owner:SOUTHWEST UNIV
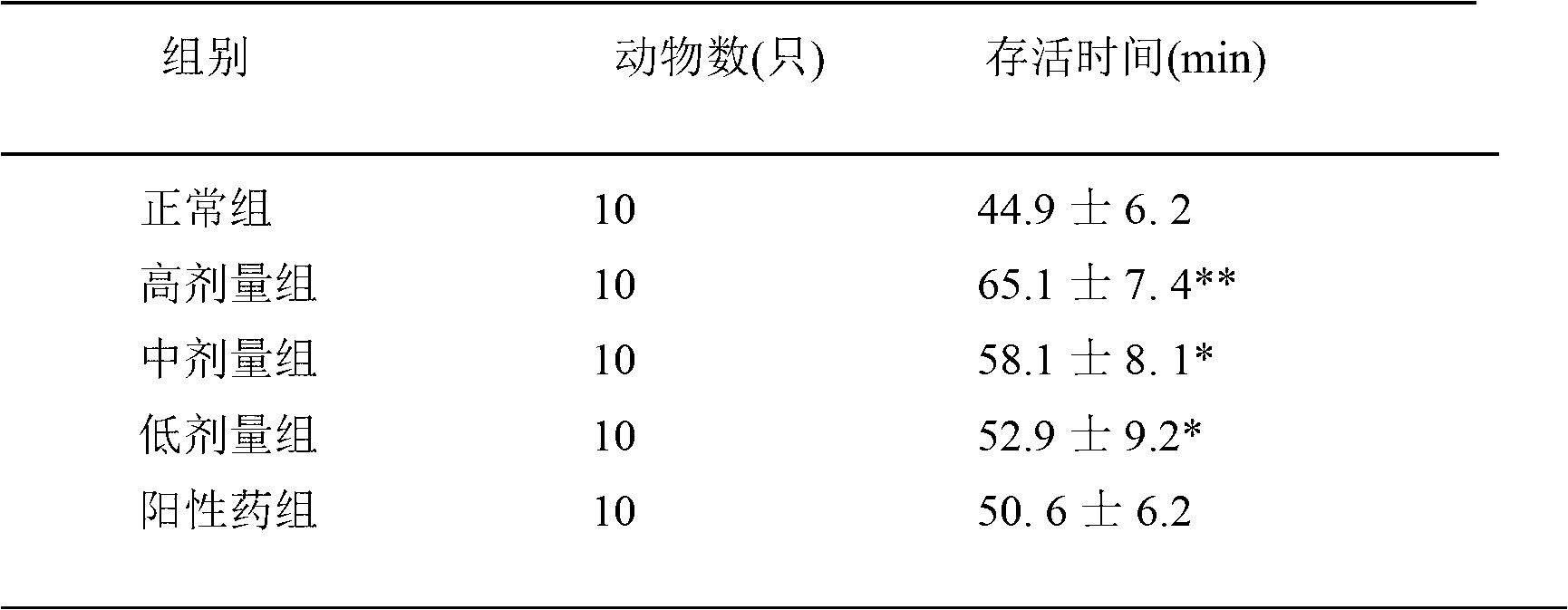
Traditional Chinese medicine composition for improving anoxia endurance as well as preparation method and application thereof
InactiveCN102935130AImprove hypoxia toleranceReasonable prescription designAnthropod material medical ingredientsAntinoxious agentsAlcoholCodonopsis pilosula
The invention discloses a traditional Chinese medicine composition for improving anoxia endurance. The traditional Chinese medicine composition comprises the following components: 10 to 30 parts of astragalus mongholicus, 20 to 40 parts of purslane speedwell herb, 15 to 25 parts of codonopsis pilosula, 10 to 30 parts of caltrop, 10 to 30 parts of asplenium antiquum makino, and 10 to 30 parts of murraya paniculata. The traditional Chinese medicine composition is in form of granula, tablet, oral liquid and capsula. The invention also discloses a preparation method of the traditional Chinese medicine composition. The preparation method comprises the following steps: adding water to decoct; concentrating; implementing alcohol precipitation; carrying out spray drying; crushing the obtained particles into dried extract powder; and adding auxiliary materials to prepare into the requested preparation. The invention also discloses application of the traditional Chinese medicine composition in preparation of medicine for improving anoxia endurance.
Owner:XINXIANG MEDICAL UNIV
Simethicone otilonium bromide chewable tablets and preparing method thereof
InactiveCN104523717AStable and controllable qualityGreat tasteAntipyreticAnalgesicsOtilonium bromideAdhesive
The invention discloses simethicone otilonium bromide chewable tablets and a preparing method of the simethicone otilonium bromide chewable tablets. The simethicone otilonium bromide chewable tablets comprise, by mass, 12.5 parts of simethicone, 4 parts of otilonium bromide, 10-40 parts of adsorbent, 25-75 parts of filler, 0.2-10 parts of adhesive, 0.2-5.0 parts of lubricant, 0.5-10 parts of corrigent, 0.05-0.5 part of aromatic and 1.5-7.5 parts of moisture sealer coating materials. According to the simethicone otilonium bromide chewable tablets, the formula is reasonable, the cost is low, and the preparing method is simple and suitable for industrial production. The prepared tablets are good in taste, the surfaces of the tablets are smooth and attractive, the tablets are even in color, appropriate in hardness, stable in quality, convenient to take and carry, and particularly suitable for the elderly, children and patients who cannot swallow solid preparations, the compliance of the patients is improved, and the simethicone otilonium bromide chewable tablets have good application prospects to treatment of the irritable bowel syndrome due to various reasons, especially the irritable bowel syndrome accompanied with abdominal distension and stomachache.
Owner:SOUTHWEST UNIVERSITY
Traditional Chinese medicine composition for treating constipation, as well as preparation method and application thereof
ActiveCN102302692BReasonable prescription designStrict compatibilityDigestive systemUnknown materialsTangerine PeelRice grain
The invention provides a traditional Chinese medicine composition for treating constipation, as well as a preparation method and application thereof. The traditional Chinese medicine composition mainly comprises the following Chinese herbal medicines in parts by weight: 18-22 parts of astragalus root, 55-65 parts of rhizoma atractylodis macrocephalae, 18-22 parts of pulvis fellis suis, 8-12 partsof fructus aurantii, 8-12 parts of dried tangerine peel, 13-17 parts of bunge cherry seed, 10-14 parts of tatarian aster root, 10-14 parts of rhizoma chuanxiong and 18-22 parts of rice-grain sprout. The traditional Chinese medicine composition can be prepared into oral liquid, tablets, capsules or granules by a corresponding preparation method. The traditional Chinese medicine composition has functions of tonifying middle-Jiao and qi and invigorating spleen for assisting spleen transportation and transformation, thus achieving the purpose of treating constipation.
Owner:HUBEI NUOKETE PHARMA
Traditional Chinese medicine composition used for treating gout, and preparation method and application thereof
ActiveCN102727753AReasonable prescription designStrict compatibilityAntipyreticAnalgesicsActive componentLicorice roots
The invention provides a traditional Chinese medicine composition. With the composition, a purpose of gout treating is achieved through heat clearing, dampness eliminating, wind-evil dispelling, and stasis removing. For achieving the purpose, the composition comprises active components of the raw materials of: 10-30 parts of Chinese lizardtail herb, 5-15 parts of common jasminorange twig and leaf, 5-15 parts of rhizoma dioscoreae hypoglaucae, 10-20 parts of appendiculate cremastra pseudobulb or common pleione pseudobulb, and 10-30 parts of licorice root. Also, a corresponding preparation method is adopted. The traditional Chinese medicine composition has a gout treating function.
Owner:BEIJING GRAND JOHAUM PHARMA CO LTD
Ibuprofen diphenhydramine orally disintegrating tablet and preparation method thereof
ActiveCN102488681BMeet quality requirementsStable and controllable qualityOrganic active ingredientsNervous disorderSodium bicarbonateOrally disintegrating tablet
The invention provides an ibuprofen diphenhydramine orally disintegrating tablet, comprising the following ingredients, by weight: 40% of ibuprofen, 5% of diphenhydramine hydrochloride or 7.6% of diphenhydramine citrate, 35-40% of filler, 6-12% of disintegrating agent, 0-5% of effervescent disintegrant, 1-2% of adhesive, 1-2.5% of surfactant, 1-1.5% of lubricant, 0.7-2.5% of sweetener and 0-0.5% of perfume. The filler is at least two from microcrystalline cellulose, mannitol and pregelatinized starch; the disintegrating agent is at least one from crospovidone, cross linked sodium carboxymethyl cellulose and sodium carboxymethyl starch; the effervescent disintegrant comprises citric acid and sodium bicarbonate in a mass ratio of 1-2:1; the adhesive is hydroxypropyl methylcellulose; the surfactant is sodium dodecyl sulfate; the lubricant is eleaostearic acid or magnesium stearate and colloid silica; the sweetener is aspartame and / or steviosin; and the perfume is medicinal essence. The invention also provides a preparation method of the orally disintegrating tablet and is simply operated, at low cost and suitable for industrialized production; and the obtained product satisfies quality requirement of an orally disintegrating tablet and has beautiful appearance, good mouthfeel and stable quality.
Owner:SOUTHWEST UNIV
A kind of traditional Chinese medicine composition for treating dysmenorrhea and its preparation method and application
InactiveCN102293894AReasonable prescription designStrict compatibilitySexual disorderPlant ingredientsMedicinal herbsChinese herbs
The invention provides a traditional Chinese medicine composition for treating dysmenorrhea, and a preparation method and application thereof. 10-20 parts of angelica, 10-20 parts of radix paeoniae alba, 4-8 parts of licorice root, 4-8 parts of rhizoma cyperi, 10-20 parts of rhizoma corydalis, 3-7 parts of cassia twig, 8-16 parts of Chinese yam, 4-8 parts of radix linderae, 8-12 parts of preparedtuber pinellia, 4-8 parts of dried orange peel, 4-8 parts of pseudo-ginseng, 2-4 parts of cinnamon and other Chinese medicinal herbs are prepared into oral liquids, tablets, capsules or granules by the corresponding preparation method. The traditional Chinese medicine composition for treating dysmenorrhea has the functions of activating blood circulation to dissipate blood stasis and alleviating pain, and is used for treating dysmenorrhea.
Owner:JIANGSU YUNYANG PHARMA GRP
Traditional Chinese medicine composition for treating coronary heart disease as well as preparation method and application thereof
InactiveCN102935195AReasonable prescription designStrict compatibilityMammal material medical ingredientsBird material medical ingredientsRemove bloodMedicine
The invention provides a traditional Chinese medicine composition for treating coronary heart disease. The traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 10 to 20 parts of poria cocos, 10 to 20 parts of root poria, 40 to 60 parts of caulis polygoni multiflori, 10 to 20 parts of rhizoma cyperi, 15 to 25 parts of rhizoma atractylodis macrocephalae, 15 to 25 parts of radix paeoniae rubra, 10 to 20 parts of radix paeoniae alba, 10 to 20 parts of pericarpium citri reticulatae, 10 to 20 parts of loranthus parasiticus, 30 to 50 parts of semen coicis, 15 to 25 parts of dogwood, 15 to 25 parts of achyranthes bidentata, 15 to 25 parts of himalayan teasel root, 30 to 50 parts of endothelium corneum gigeriae galli, 15 to 25 parts of radix aucklandiae, 10 to 20 parts of radix bupleuri, 20 to 30 parts of malt, and 10 to 20 parts of liquorice. The traditional Chinese medicine composition has the effects of promoting blood circulation to remove blood stasis, and promoting Qi circulation to relieve pain, so that the purpose of treating coronary heart disease can be achieved. The invention also discloses a preparation method and application of the traditional Chinese medicine composition.
Owner:王颖翠
Chinese medicinal composition for treating haemorrhoids, and preparation method and application thereof
InactiveCN102335343ARich sources of medicineEasy to get materialsHydroxy compound active ingredientsCardiovascular disorderBletilla striataBULK ACTIVE INGREDIENT
The invention provides a Chinese medicinal composition. The Chinese medicinal composition fulfils the aims of treating haemorrhoids by easing pain, diminishing inflammation, and improving microcirculation. To fulfill the aim, the adopted technical scheme is that: the Chinese medicinal composition is prepared from the following active ingredients in part by weight: 30 to 50 parts of asparagus juice, 10 to 30 parts of bletilla striata, 30 to 50 parts of Japanese knotweed, 3 to 7 parts of dragon's blood, 3 to 7 parts of pearl powder, and 1 to 3 parts of borneol; and the corresponding preparationmethod is adopted. The Chinese medicinal composition has the effects of easing pain, diminishing inflammation, improving microcirculation and treating haemorrhoids.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Traditional Chinese medicine composition for treating gall-stone as well as preparation method and application
InactiveCN104436001AReasonable prescription designStrict compatibilityDigestive systemPill deliveryCurcuma aromaticaScutellaria baicalensis
The invention provides a traditional Chinese medicine composition for treating gall-stone. The traditional Chinese medicine composition for treating gall-stone is prepared by extracting the following raw material medicines in parts by weight: 10-20 parts of red gentian, 10-20 parts of campylotropis hirtella, 4-8 parts of radix bupleuri, 4-8 parts of scutellaria baicalensis, 10-20 parts of fructus aurantii, 3-7 parts of curcuma aromatica and 8-16 parts of rhizoma cyperi. The traditional Chinese medicine composition for treating gall-stone is capable of clearing heat and diminishing inflammation, regulating qi and alleviating pain, drying damp and strengthening spleen, promoting circulation and removing stasis, and treating gall-stone. The invention further discloses a preparation method and an application.
Owner:NANJING HUAKUANG INFORMATION CONSULTING CENT
Chinese medicinal composition for resisting fatigue, and preparation method and application thereof
ActiveCN102614372ARelieve physical fatigueReasonable prescription designAntinoxious agentsPlant ingredientsPatriniaAlcohol
The invention discloses a Chinese medicinal composition for resisting fatigue. The Chinese medicinal composition contains the following components in part by weight: 10 to 30 parts of integripetal rhodiola herb, 20 to 40 parts of sargentgloryvine stem, 5 to 10 parts of dwarf lilyturf tuber, 10 to 30 parts of diversifolious patrinia root and 10 to 30 parts of common jasminorange leaf and twig, wherein the Chinese medicinal composition may be granules, tablets, oral liquid and capsules. The invention also discloses a preparation method for the Chinese medicinal composition. The method comprisesthe following steps of: decocting in water, concentrating, performing alcohol precipitation, spray-drying, crushing the obtained granules into dry extract powder, and adding auxiliary materials to prepare the required preparation. The invention also discloses application of the Chinese medicinal composition to the preparation of medicines with a fatigue-resisting effect.
Owner:BEIJING BEILU PHARM CO LTD
A kind of deer blood medicinal wine for treating rheumatoid arthritis
InactiveCN102258589AReasonable prescription designStrict compatibilityAnthropod material medical ingredientsAntipyreticCitrate sodiumCentipede
The invention provides a deer blood medicinal wine for treating rheumatoid arthritis, which mainly comprises the following steps: dissolving deer blood in 3.8% sodium citrate solution; removing impurities from antler, deer bone, scorpion, centipede, angelica, black-tail snake, hemlock parsley, szechuan aconite, safflower, ephedra, cassia twig, heracleum hemsleyanum michaux and radix achyranthis bidentatae, pulverizing, and passing through a 60-mesh screen; and soaking the pulverized Chinese herbal medicines and the deer blood solution in 30-50 vol% white spirit for 10-20 days, and filtering to obtain the deer blood medicinal wine for treating rheumatoid arthritis. The deer blood medicinal wine can quickly and effectively treat rheumatoid arthritis, has the advantages of simple formula andlow cost, is easy to produce, and is suitable for the demands of most patients with rheumatoid arthritis; the rheumatoid arthritis can not easily relapse; and the deer blood can not easily solidify.
Owner:淮安市淮安区古神梅花鹿养殖场
Health medicinal liquor containing cibotium barometz and preparation method of health medicinal liquor
InactiveCN104435615APromote absorptionGreat tasteDispersion deliveryDigestive systemParnassiaBULK ACTIVE INGREDIENT
The invention provides health medicinal liquor containing cibotium barometz. The active ingredient of the medicinal liquor is prepared from the following medicinal raw materials in parts by weight: cibotium barometz, leafy parnassia, polygonum multiflorum and the like, as well as 5000-10000 parts of 30-50-degree distilled spirit. The medicinal liquor is prepared by the following steps: removing impurities, and grinding; filtering through a 60-mesh screen; soaking the medicinal materials in 30-50-degree distilled spirit for 10-20 days; and filtering medicinal residues to obtain the medicinal liquor. The medicinal liquor has a health effect, and is simple in formula, low in cost and easy to produce.
Owner:NANJING HUAKUANG INFORMATION CONSULTING CENT
Ointment for treating haemorrhoids as well as preparation method and application thereof
InactiveCN104013672AGood hemostatic effectSignificant effectAerosol deliveryOintment deliveryMotherwortMedicine
The invention provides an ointment for treating haemorrhoids. The active component contained in the ointment is prepared from the following raw materials in parts by weight: 10-20 parts of red musketeers, 10-30 parts of wild dam artemisia, 10-15 parts of motherwort and 3-7 parts of pearl powder. The target of treating the haemorrhoids is achieved by astringing to stop bleeding and relieving pain. The invention also discloses a preparation method and use thereof.
Owner:四川中科佰氏制药有限公司
Ibuprofen diphenhydramine dispersing tablet and preparation method thereof
ActiveCN102488680AReasonable prescription designSimple and fast operationOrganic active ingredientsNervous disorderActive agentDiphenhydramine hydrochloride
The invention provides an ibuprofen diphenhydramine dispersing tablet, which consists of the following components in percentage by mass: 40 percent of ibuprofen, 5 percent of diphenhydramine hydrochloride or 7.6 percent of diphenhydramine citrate, 30-43 percent of a filler, 5-15 percent of a disintegrant, 2-8 percent of a bonding agent, 0.1-1 percent of a surfactant, 1.5-4 percent of a lubricating agent and 0.5-1.5 percent of a flavoring agent, wherein the filler is at least two of microcrystalline cellulose, pre-gelatinized starch, lactose and mannite; the disintegrant is cross-linked sodiumcarboxymethylcellulose and / or cross-linked polyvidone; the bonding agent is polyvidone K30; the surfactant is sodium dodecyl sulfate; the lubricating agent is magnesium stearate and superfine silica gel powder; and the flavoring agent is aspartame and steviosin. The invention further provides a preparation method of the dispersing tablet. The preparation method is easy and convenient to operate, has low cost, and is suitable for industrial production. An obtained product has stable and controllable quality, attractive appearance, good mouthfeel and proper hardness, is fully disintegrated in water at the temperature of 20+ / -1 DEG C within 3 minutes, and passes through a No.2 sieve; and the medicament dissolvability is over 85 percent 30 minutes later.
Owner:SOUTHWEST UNIV
Foot soaking powder for relaxing tendons and collaterals and strengthening muscles and bones and preparation method of foot soaking powder
InactiveCN103919877AWide variety of sourcesReasonable prescription designPowder deliverySkeletal disorderDiseaseBlood capillary
The invention belongs to the technical field of traditional Chinese medicine, and particularly relates to foot soaking powder for relaxing tendons and collaterals and strengthening muscles and bones and a preparation method of the foot soaking powder. The foot soaking powder for relaxing tendons and collaterals and strengthening muscles and bones disclosed by the invention consists of papaya, parabarium micranthum, suberect spatholobus stem and Chinese starjasmine stem. The foot soaking powder penetrates into skin under the thermal assistance of hot water, is absorbed by foot blood capillaries, and enters a human body blood circulating system, so that the disease-curing and health-preserving effects of improving physical fitness, regulating body, relaxing tendons and collaterals, strengthening muscles and bones and the like are achieved.
Owner:李万水
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com