Compound vitamin (3) pharmaceutical composition for injection and preparation method thereof
A technology for compound vitamins and vitamins, which is applied in the directions of drug combinations, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc., can solve the problems affecting product quality and safety, the stability of active ingredients, etc. The effect of stable and controllable, easy preparation method and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Each 1000 vials of freeze-dried powder injection contains the following components:
[0028] Vitamin B 1 10g
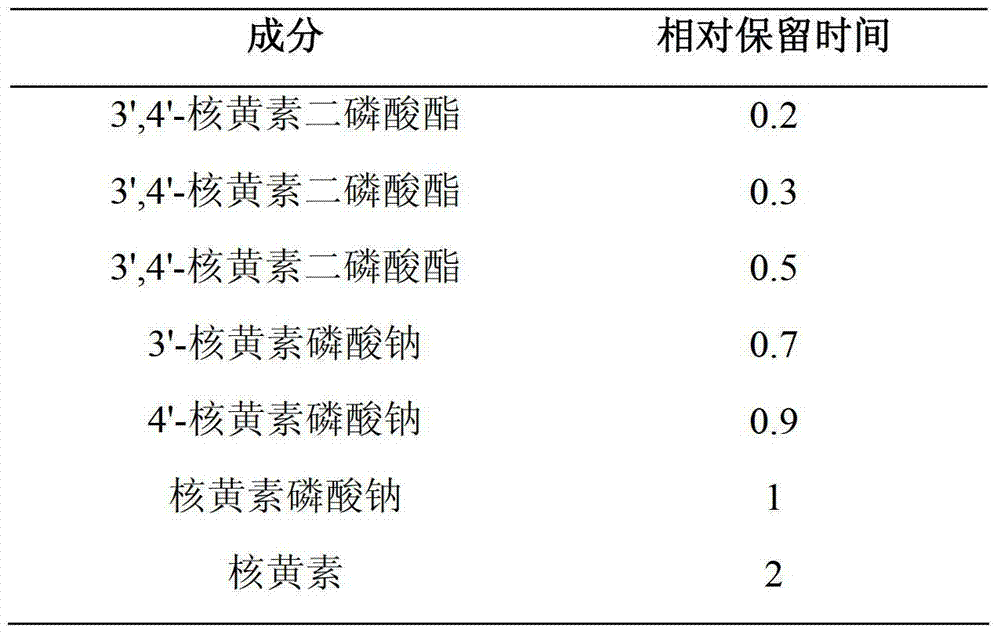
[0029] Riboflavin sodium phosphate 6.355g (calculated after deducting crystal water)
[0030] Vitamin C200g
[0031] Glycine 400g
[0032] Thiourea 6.2g
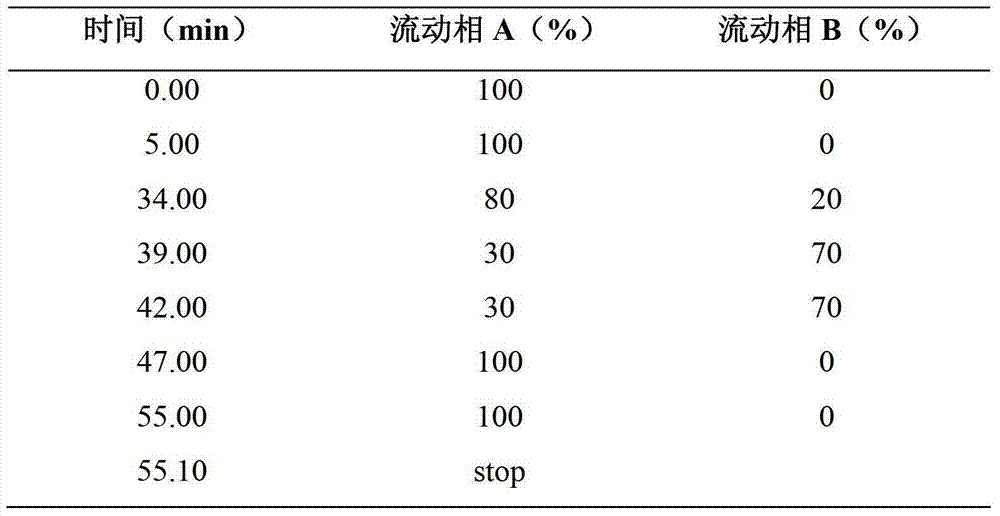
[0033] Weigh glycine, add water for injection to prepare 20% glycine solution, and then weigh vitamin B 1 , Riboflavin Sodium Phosphate, and Vitamin C were added to glycine solution and stirred to dissolve in sequence, and water for injection was added to 90% of the total liquid volume of 3000ml, and stirred evenly; 0.03% (g / ml) activated carbon for needles was added, and nitrogen was filled to stir and adsorb After 15 minutes, decarbonize and filter; add water for injection to the filtrate until the total volume of the solution is 3000ml, filter through a 0.22μm filter, and aseptically fill the filtrate into 1000 vials to be freeze-dried.
[0034]Freeze-drying: When the temperature of the shelf drops t...
Embodiment 2
[0036] Each 1000 vials of freeze-dried powder injection contains the following components:
[0037] Vitamin B 1 10g
[0038] Riboflavin sodium phosphate 6.355g (calculated after deducting crystal water)
[0039] Vitamin C200g
[0040] Glycine 450g
[0041] Thiourea 6.2g
[0042] Weigh glycine, add water for injection to prepare 10% glycine solution, and then weigh vitamin B 1 , riboflavin sodium phosphate, vitamin C, add in glycine solution and stir to dissolve in turn, add water for injection to 80% of the total liquid volume of 3000ml, stir evenly; add 0.03% (g / ml) activated carbon for needles, fill with nitrogen and stir for adsorption After 15 minutes, decarbonize and filter; add water for injection to the filtrate until the total volume of the solution is 3000ml, filter through a 0.22μm filter, and aseptically fill the filtrate into 1000 vials to be freeze-dried.
[0043] Freeze-drying: When the temperature of the shelf drops to -45°C and the temperature of the prod...
Embodiment 3
[0045] Each 1000 vials of freeze-dried powder injection contains the following components:
[0046] Vitamin B 1 18g
[0047] Riboflavin sodium phosphate 9g (calculated after deducting crystal water)
[0048] Vitamin C270g
[0049] Glycine 500g
[0050] Thiourea 8g
[0051] Weigh glycine, add water for injection to prepare 35% glycine solution, and then weigh vitamin B 1 , riboflavin sodium phosphate, vitamin C, add in glycine solution and stir to dissolve in turn, add water for injection to 90% of the total liquid volume of 3000ml, stir evenly; add 0.03% (g / ml) activated carbon for needles, fill with nitrogen and stir for adsorption After 15 minutes, decarbonize and filter; add water for injection to the filtrate until the total volume of the solution is 3000ml, filter through a 0.22μm filter, and aseptically fill the filtrate into 1000 vials to be freeze-dried.
[0052] Freeze-drying: When the temperature of the shelf drops to -45°C and the temperature of the product dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com