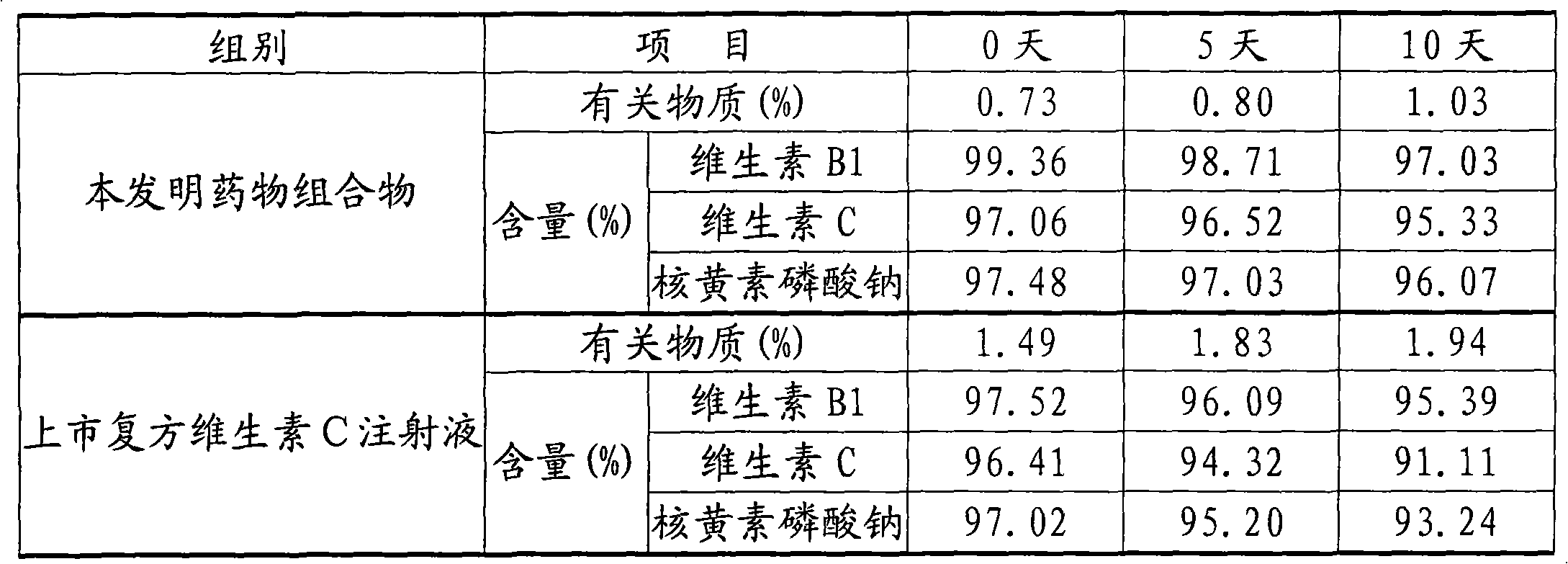
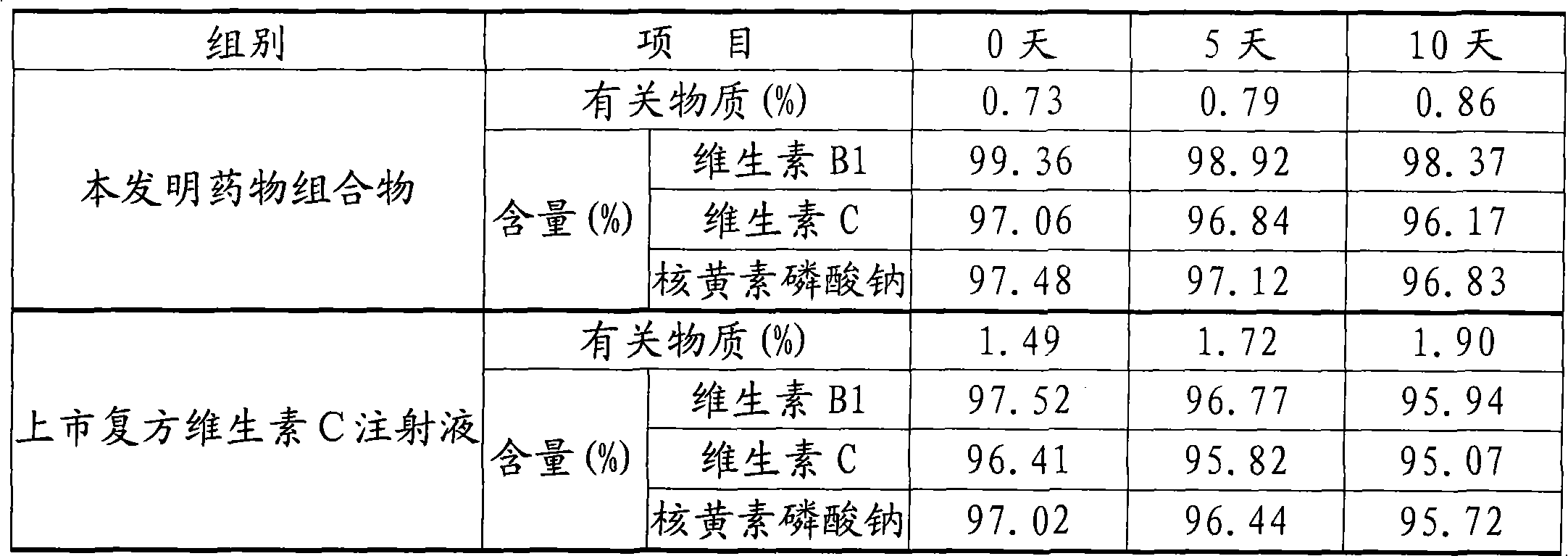
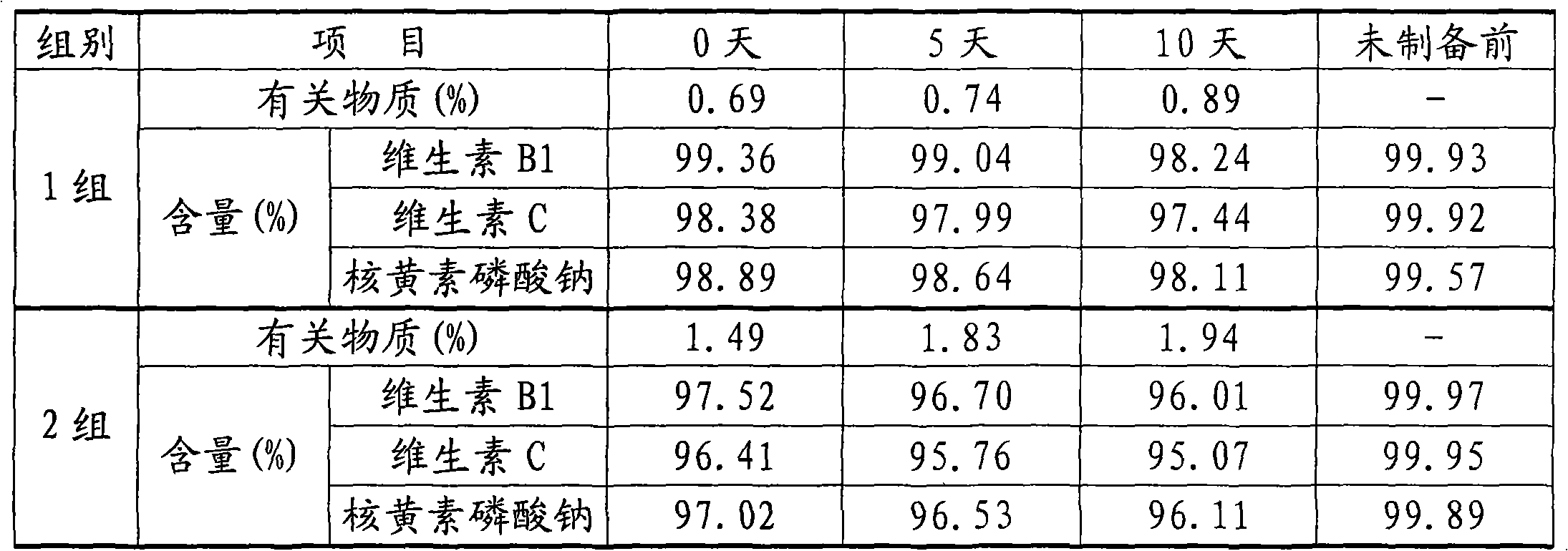
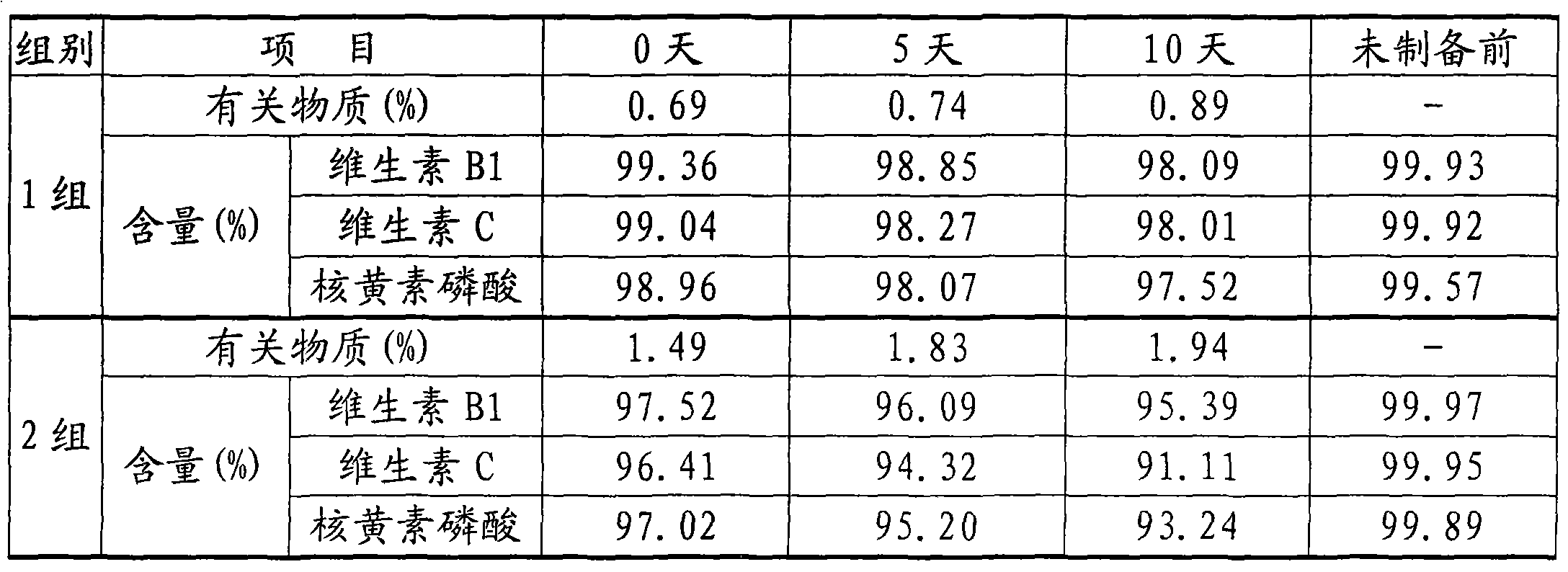
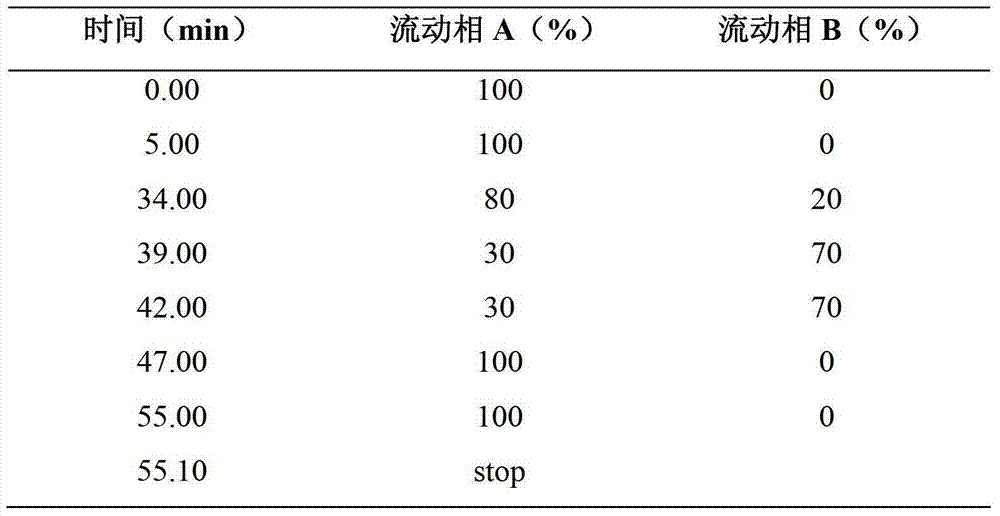
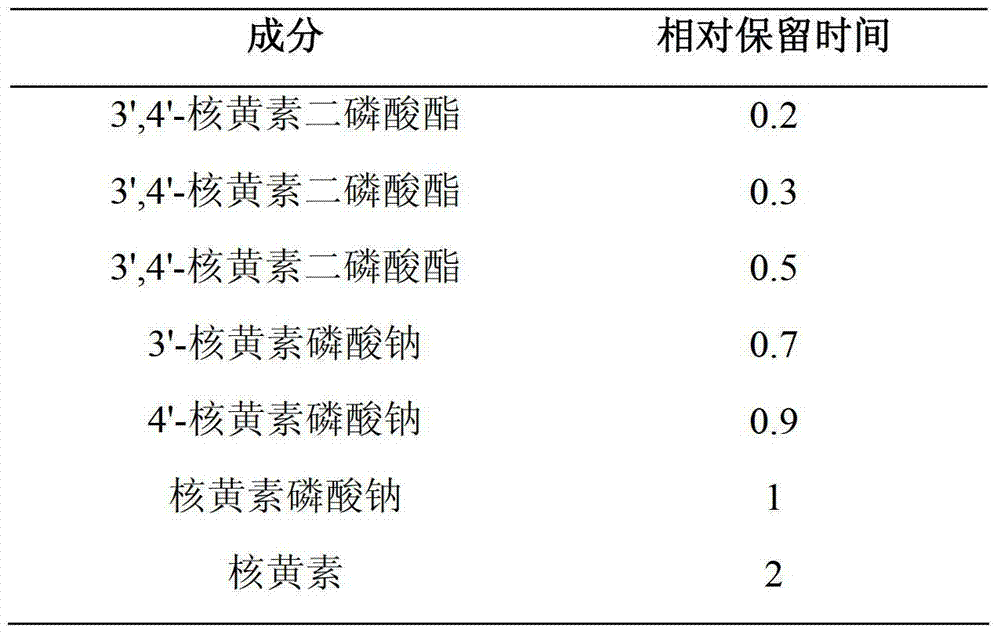
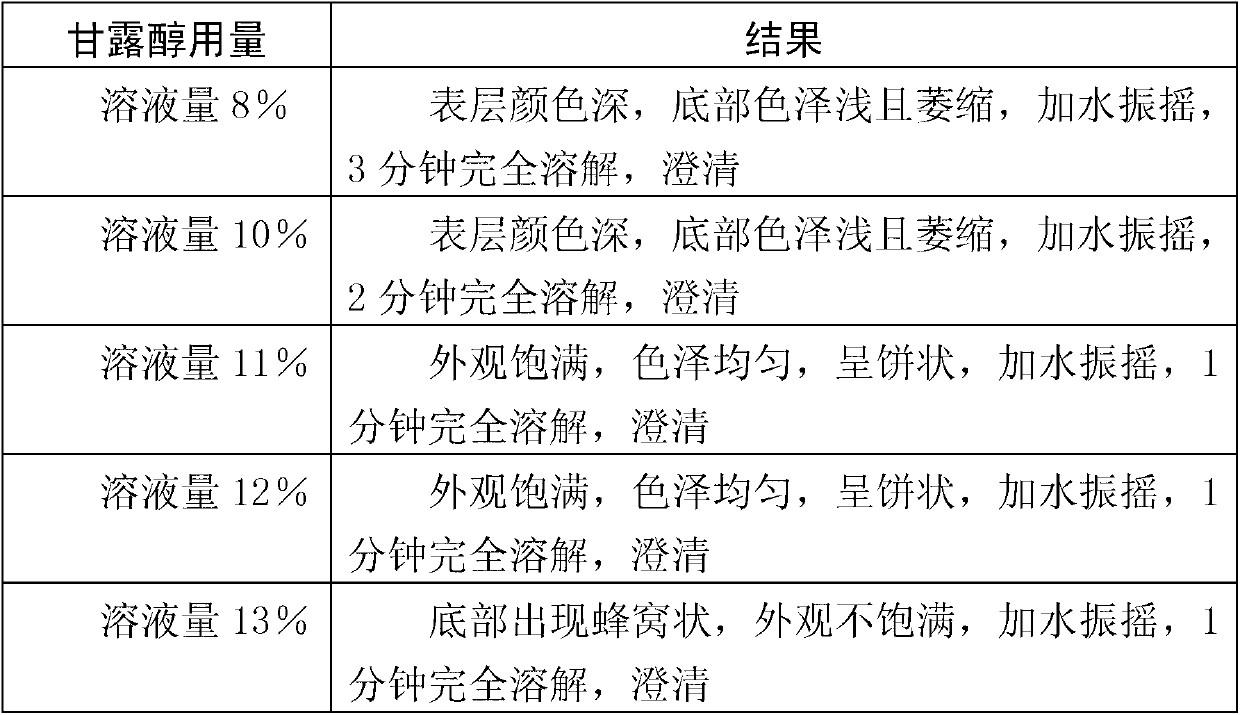
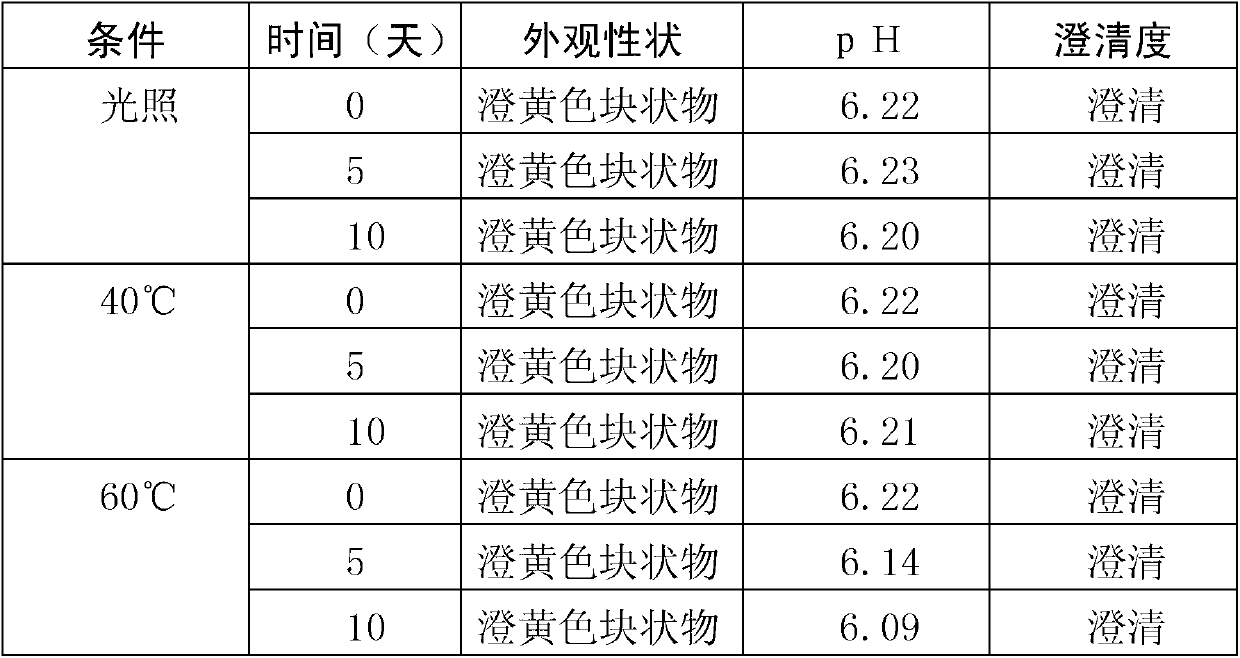
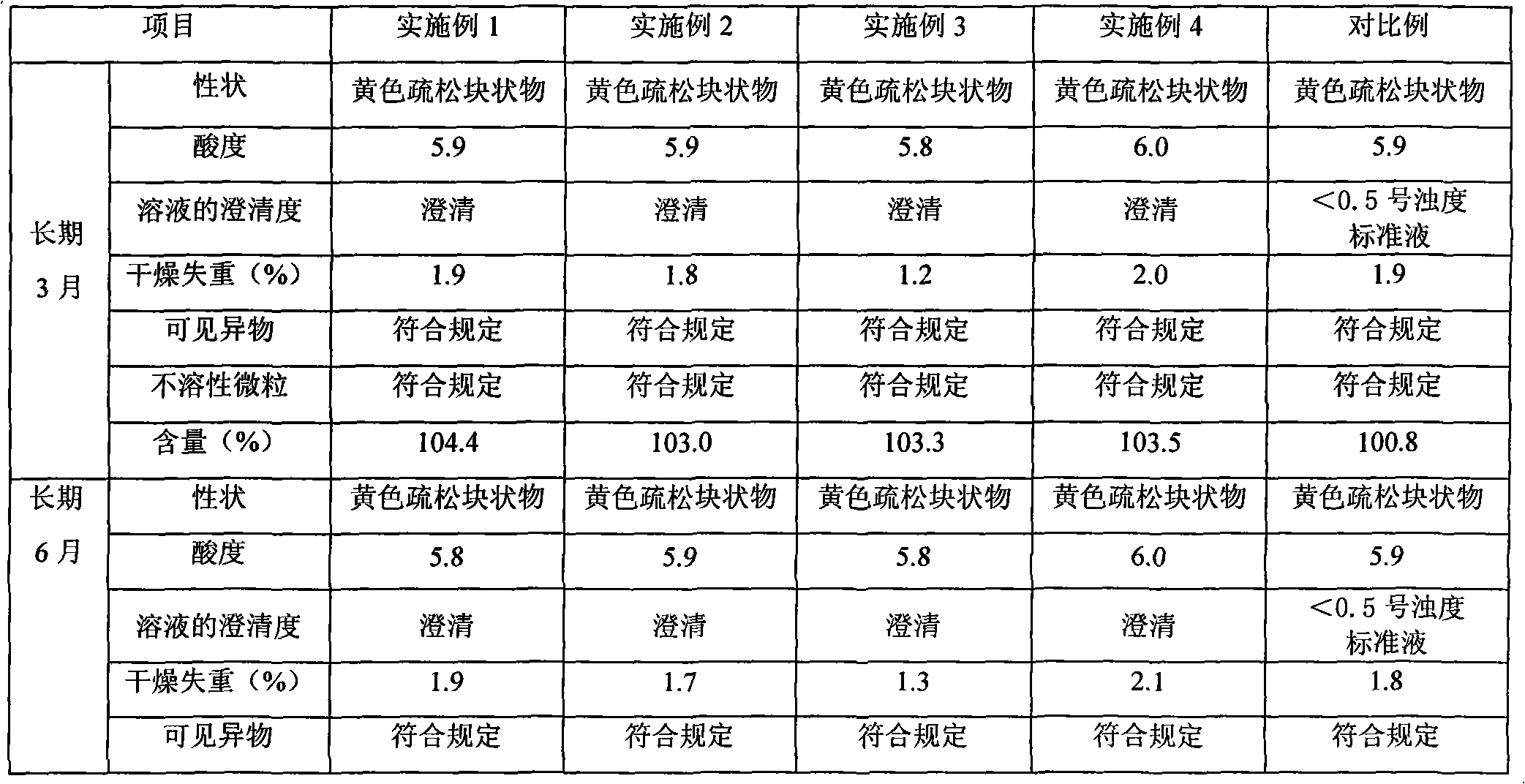
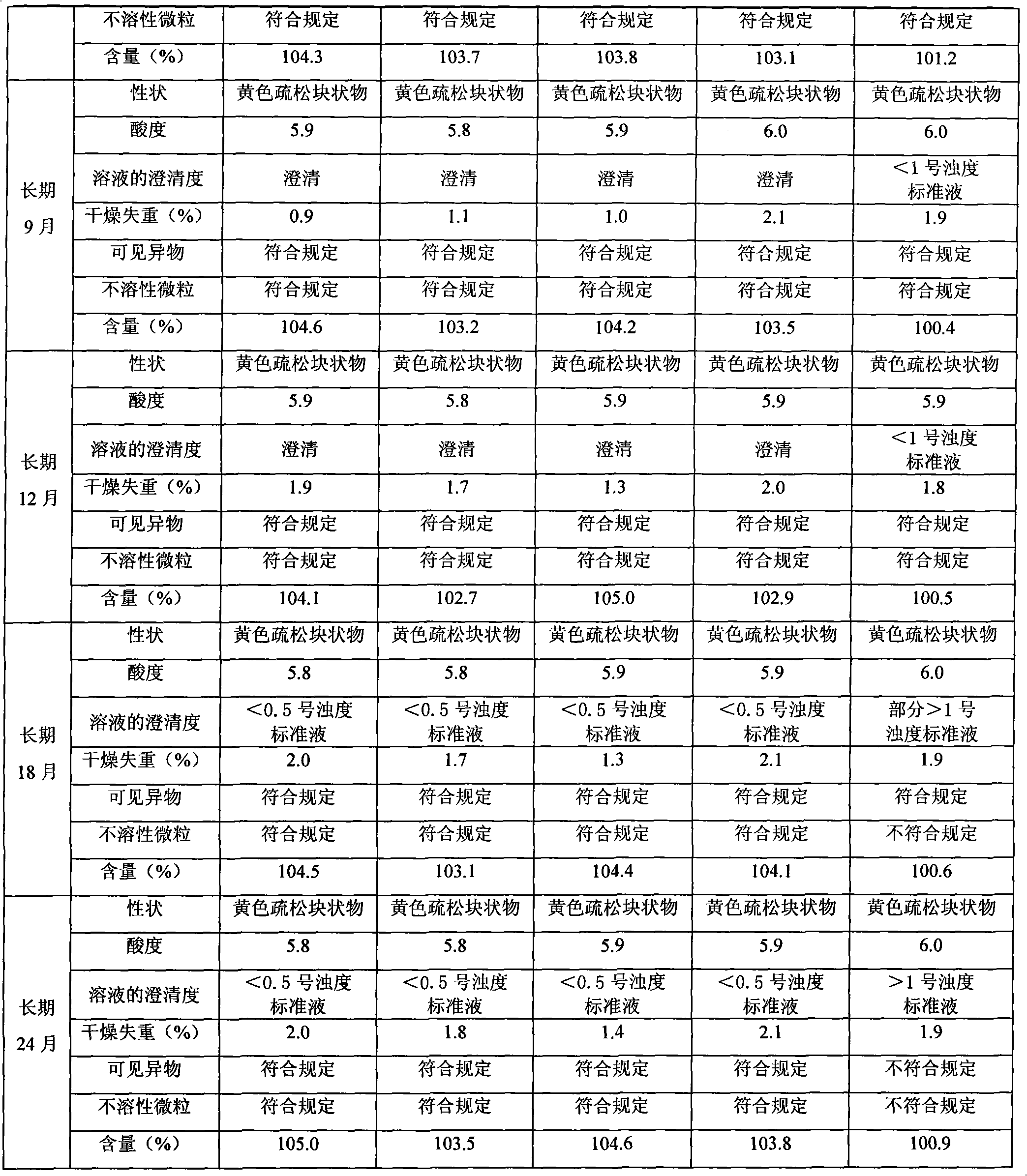
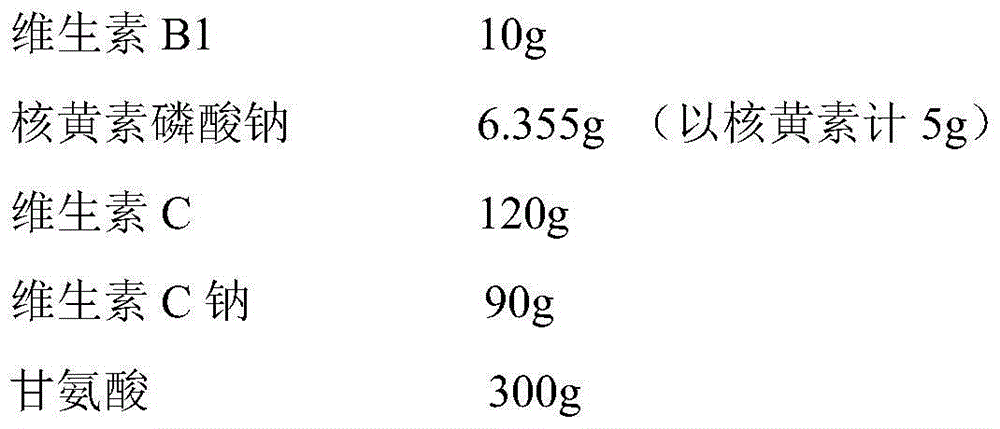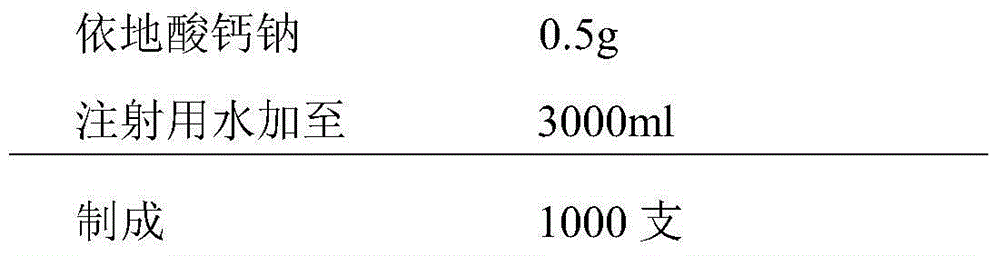Patents
Literature
65 results about "Riboflavin sodium phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
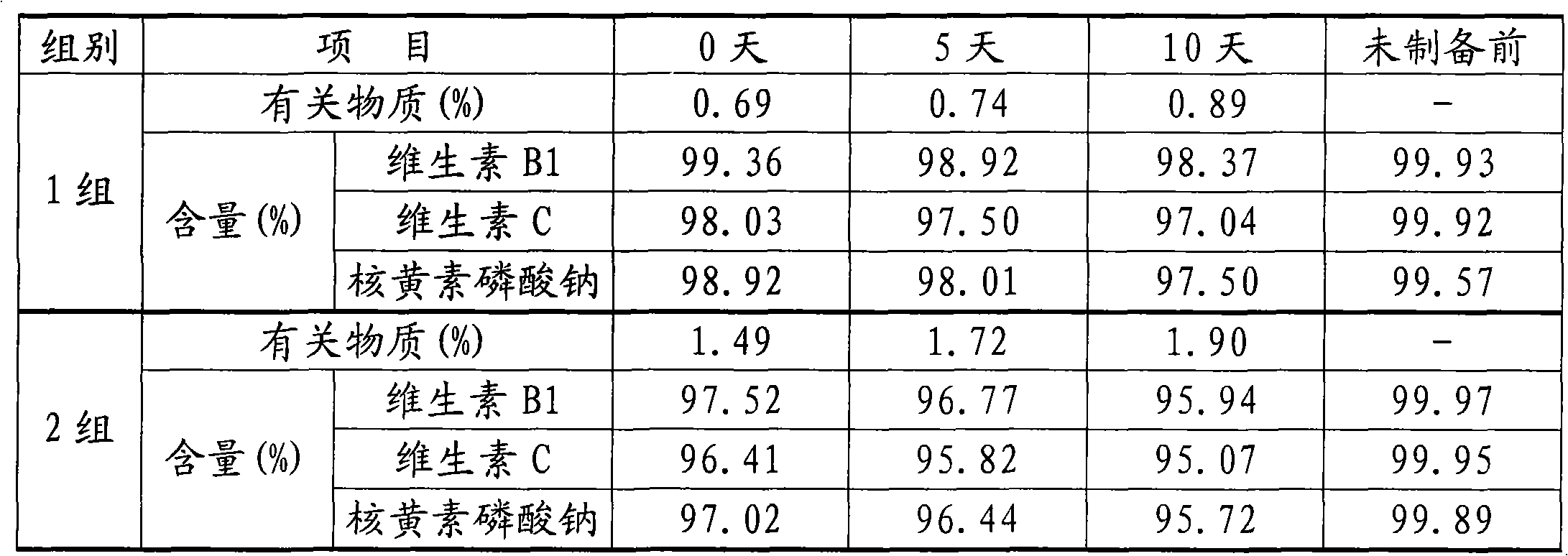
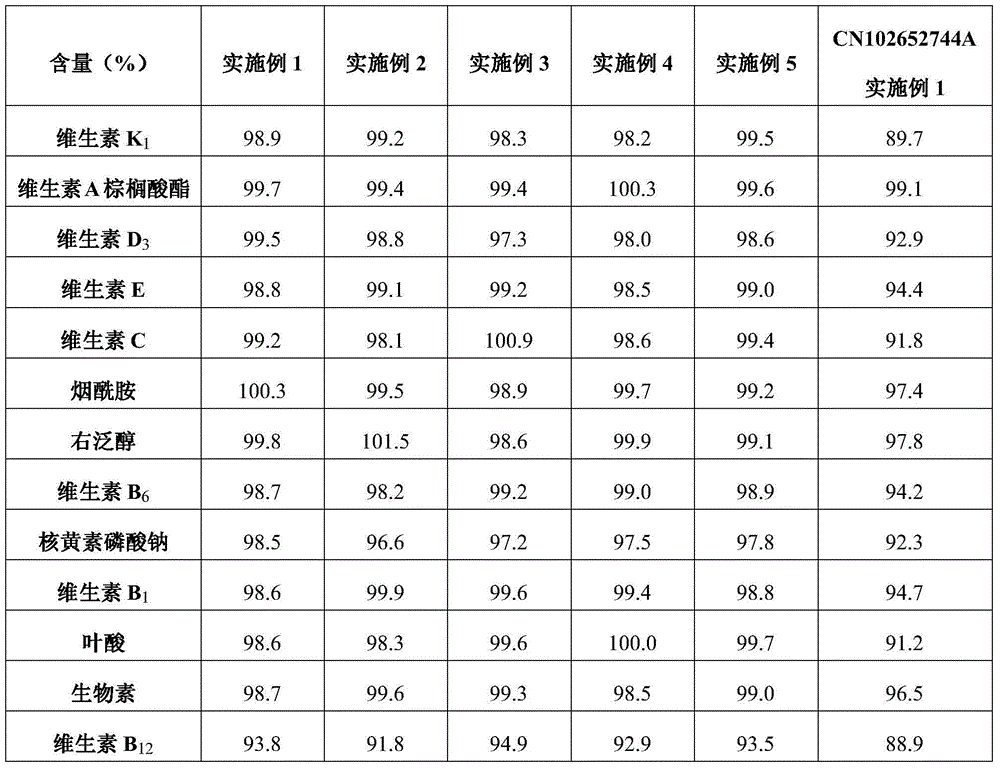
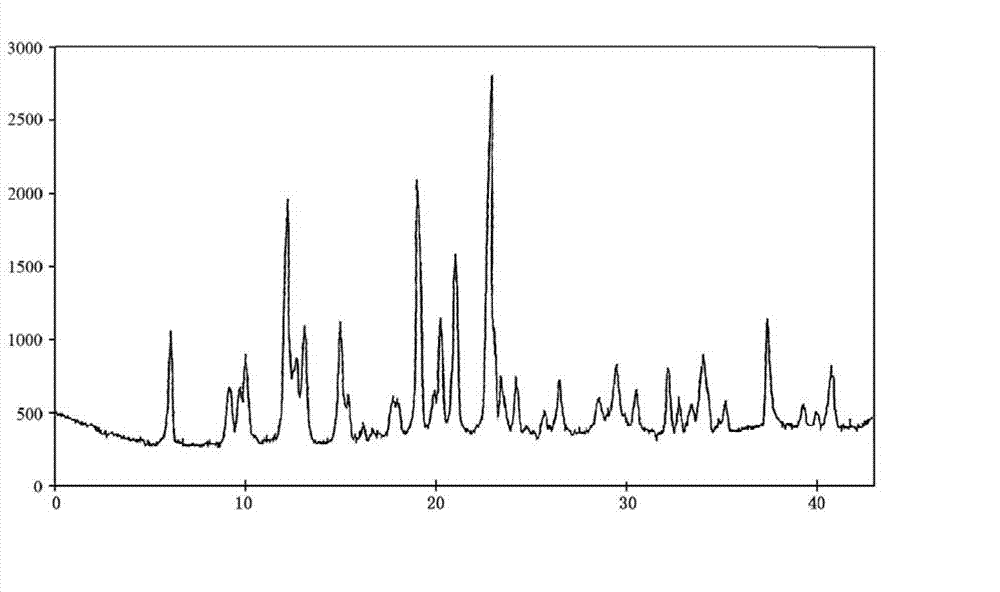
Content analysis and detection method of twelve compound vitamins for injection
InactiveCN106153796AImprove detection efficiencyHigh detection sensitivityComponent separationAlpha-TocopherolVitamin B12
The invention discloses a content analysis and detection method of twelve compound vitamins for injection. Liposoluble components including vitamin A palmitate, vitamin D3 and racemic alpha-tocopherol are detected under the same high-performance liquid phase method conditions, a test solution is prepared by extracting a preparation content by non-polar organic solvent, and the detection wavelength is 265+ / -3nm. Water-soluble components including cocarboxylase tetrahydrate, riboflavin sodium phosphate, vitamin B6, vitamin C, nicotinamide, folic acid and dexpanthenol are detected under the same high-performance liquid phase method conditions, and the detection wavelength is 210+ / -3nm. Biotin and vitamin B12 are subjected to high-performance liquid phase method based detection, the detection wavelength is 200-600nm, and the preferable detection wavelength for the vitamin B12 is 550+ / -3nm. The method has high applicability.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
Compound vitamin (3) freeze-dried powder injection for injection and preparation method and analysis method thereof
ActiveCN102552294ATransportation advantageImprove stabilityPowder deliveryComponent separationGlycineFreeze-drying
The invention provides a compound vitamin freeze-dried powder injection for injection, which comprises vitamin B15-15 parts by weight, riboflavin sodium phosphate 3-8 parts by weight (counting by reducing crystal water), vitamin C 150-250 parts by weight, glycine 350-500 parts by weight and water for injection 1000-5000 parts by weight which is used as a solvent during preparation and is finally removed. The compound vitamin freeze-dried powder injection for injection is good in stability, high in safety and convenient to store and transport. The invention further provides a preparation method of the compound vitamin freeze-dried powder injection for injection. The compound vitamin freeze-dried powder injection for injection prepared through the preparation method is good in stability and high in safety. The invention further provides an analysis method of the compound vitamin freeze-dried powder injection for injection, and the analysis method is particularly specific to relevant substance of unstable basic medicines.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Porcine reproductive and respiratory syndrome virus diluent and preparation method thereof
InactiveCN105505887APromote growthGuaranteed normal growthSsRNA viruses positive-senseMicroorganism based processesCymbopogon distansPhosphate
The invention provides a porcine and respiratory syndrome virus diluent and a preparation method thereof and belongs to the technical field of veterinary biological products. Every 1000 ml phosphate buffer of the diluent is prepared from, by weight, 10-30 g of amino acid combination, 10-30 g of compound traditional Chinese medicine polysaccharides and 10-30 g of liposome, wherein the amino acid combination comprises folic acid, riboflavin sodium phosphate, dexpanthenol and ascorbic acid, and the compound traditional Chinese medicine polysaccharides are extracted from pubescent angelica roots, Cymbopogon distans (Nees)A. Camus, chastetree fruit, butterflybush flowers, radix scrophulariae and red peony roots. The diluent has the advantages that after virus inoculation, cell growth can be promoted, and normal growth of cells can be maintained; viruses can infect the cells more easily after processing, and the probability that the viruses infect the cells is greatly increased; virus titer can be greatly increased in the same culture time, and virus content is increased by 10-100 times.
Owner:浙江美保龙生物技术有限公司
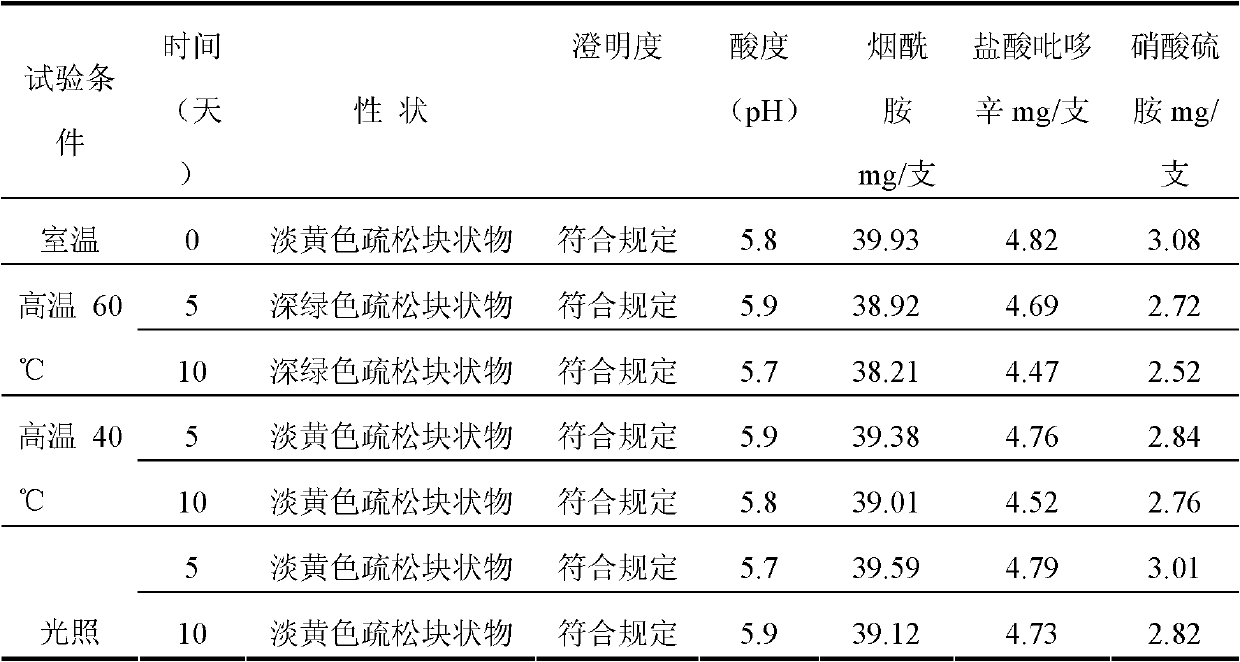
Medical composition for injection
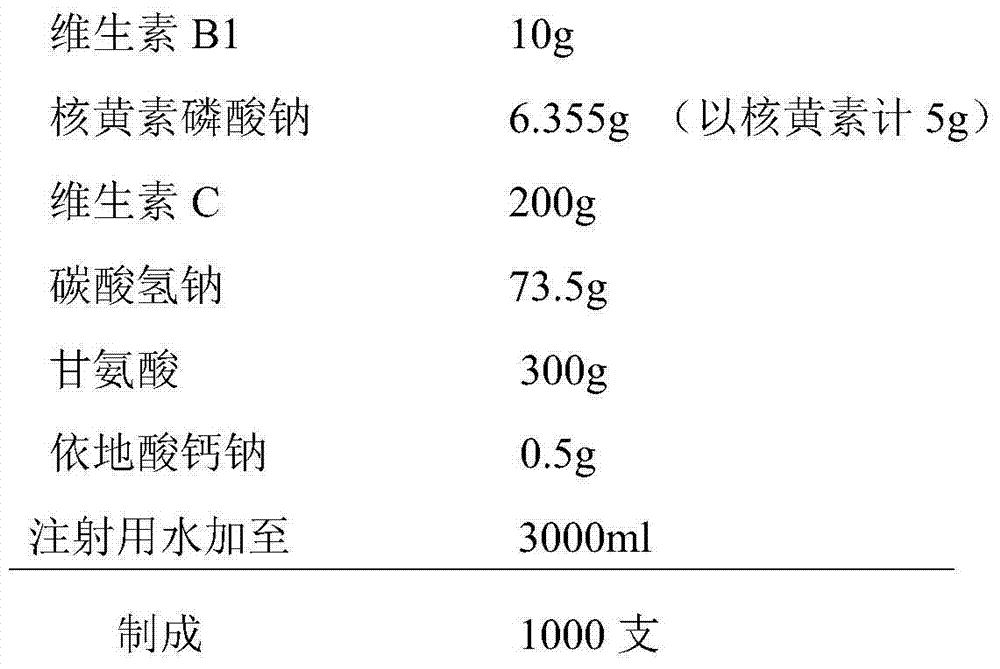
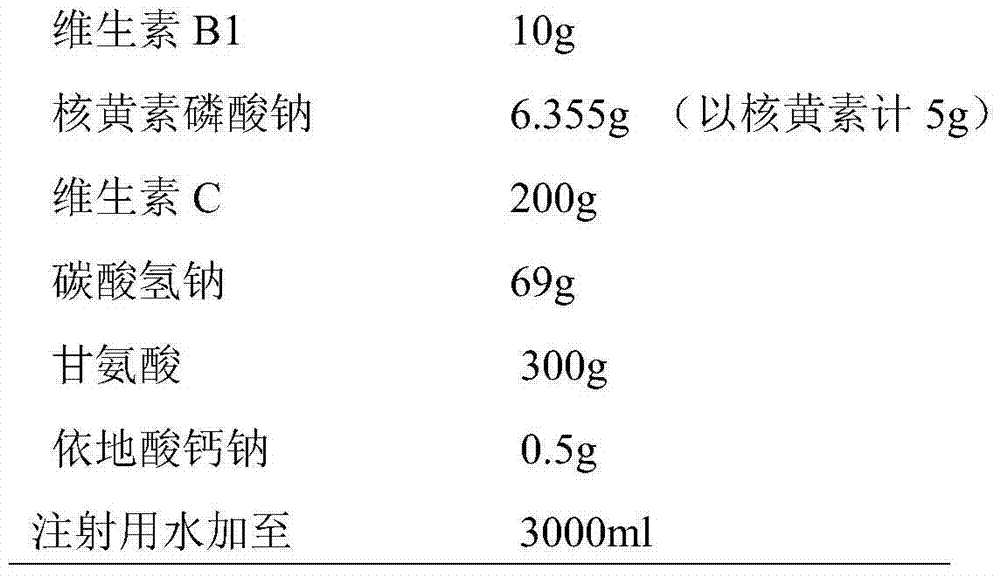
The invention discloses a medical composition for injection, which is characterized by comprising the following components by weight part: 5-20 parts of vitamin B1, 1-10 parts of riboflavin sodium phosphate (metered by riboflavin), 100-300 parts of vitamin C, 100-500 parts of excipient, 0.1-1 part of complexing agent and a pH regulating agent. Compared with a vitamin C injection on the market, the medical composition has low content of related substances and high content of effective constituents; and proved by high-temperature, high-humidity and strong-light tests, the medical composition has better stability.
Owner:北京普瑞博思生物科技有限公司
Medical composition of compound vitamin C injection
InactiveCN101623284AMetabolism disorderPharmaceutical product form changeHigh humidityChemical composition
The invention discloses a medical composition of a compound vitamin C injection, which comprises the following raw materials by weight part: 5-20 parts of vitamin B1, 1-10 parts of riboflavin sodium phosphate (metered by riboflavin) and 100-300 parts of vitamin C. The medical composition is characterized in that the vitamin C is mixed with cation exchange resin to obtain vitamin C sodium, and the vitamin C sodium, the vitamin B1 and the riboflavin sodium phosphate are prepared into an injection. Compared with a compound vitamin C injection on the market, medical composition has less reduction of effective constituents such as the vitamin C, and the like in a preparation process and has low content of related substances and high content of the effective constituents. Proved by high-temperature, high-humidity and strong-light tests, the compound vitamin C injection has better stability.
Owner:北京普瑞博思生物科技有限公司
Water-soluble vitamin composition freeze-drying preparation for injection
ActiveCN101904862AGranularity adjustableConcentrated particle size distributionPowder deliveryMetabolism disorderFreeze-dryingSodium pantothenate
The invention discloses a water-soluble vitamin composition freeze-drying preparation for injection. The freeze-drying preparation comprises the following raw materials and is prepared into 1,000 bottles: 2.8 to 3.4 grams of thiamine mononitrate, 36 to 44 grams of nicotinamide, 4.4 to 5.4 grams of pyridoxine hydrochloride, 14.8 to 18.1 grams of sodium pantothenate, 4.4 to 5.4 grams of riboflavin sodium phosphate, 102 to 124 grams of sodium vitamin C, 54 to 66 milligrams of biotin, 0.36 to 0.44 gram of folic acid, 124.5 to 6.0 milligrams of vitamin B, and 0.4 to 0.6 gram of methyl-p-hydroxy benzoate, wherein the nicotinamide is nicotinamide hydrate, and the sodium pantothenate is sodium pantothenate hydrate. The water-soluble vitamin for injection has adjustable grain size, concentrated grain size distribution, glabrous surface, high product fluidity, greatly improved stability and high dissolution rate, and a preparation process of the preparation is simple and is favorable for popularization and use.
Owner:SHANDONG YUXIN PHARMA CO LTD
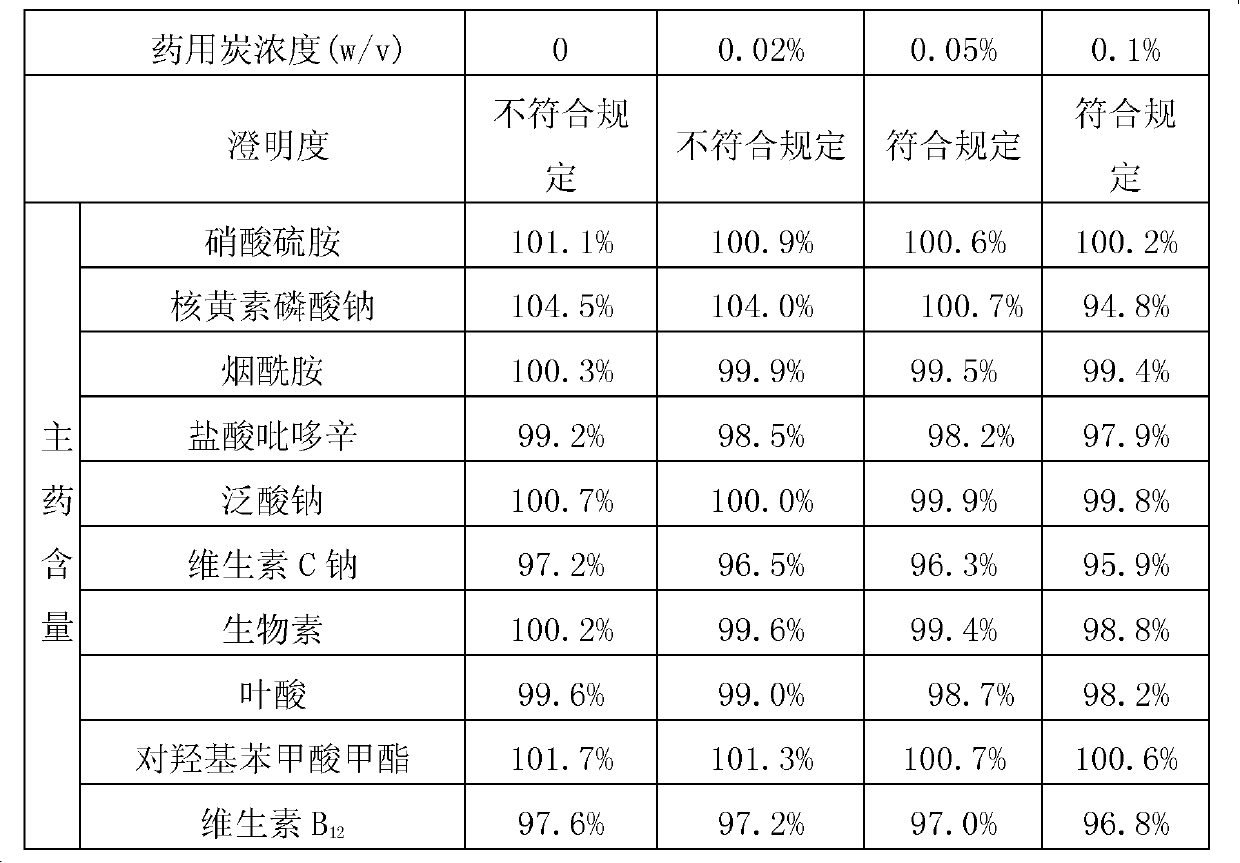
Compound vitamin (3) pharmaceutical composition for injection and preparation method thereof
InactiveCN103202844APlay an antioxidant roleNot easy to decomposePowder deliveryMetabolism disorderGlycineActivated carbon
The invention provides a compound vitamin (3) pharmaceutical composition for injection and a preparation method thereof. The composition is powder injection and comprises vitamin B1, riboflavin sodium phosphate (namely vitamin B2 sodium phosphate), vitamin C, stabilizer glycine and antioxidant thiourea. The preparation method comprises the following steps: preparing liquid medicine, performing activated carbon adsorption, decarburizing, filtering and degerming, filling and performing freeze-drying. The compound vitamin (3) pharmaceutical composition for injection does not contain a pH regulator and is high in stability and high in safety.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Pharmaceutical composition of 12 complex vitamins for injection and preparation method thereof
InactiveCN103006683AHydroxy compound active ingredientsMetabolism disorderThiamine pyrophosphateAlpha-Tocopherol
The invention provides a pharmaceutical composition of 12 complex vitamins for injection and a preparation method thereof. The pharmaceutical composition of 12 complex vitamins for injection provided by the invention comprises the active ingredients of vitamin A palmitate, cholecalciferol, racemic alpha-tocopherol, ascorbic acid, nicotinamide, dexpanthenol, pyridoxine hydrochloride, riboflavin sodium phosphate, tetrahydrate thiamine pyrophosphate, folic acid, D-biotin and cyanocobalamin, and auxiliary materials namely polysorbate 80 and mannitol. The prescription provided by the invention does not contain auxiliary material glycocholic acid, and can be clinically used for people with over-high glycocholic acid.
Owner:SHANXI PUDE PHARMA CO LTD
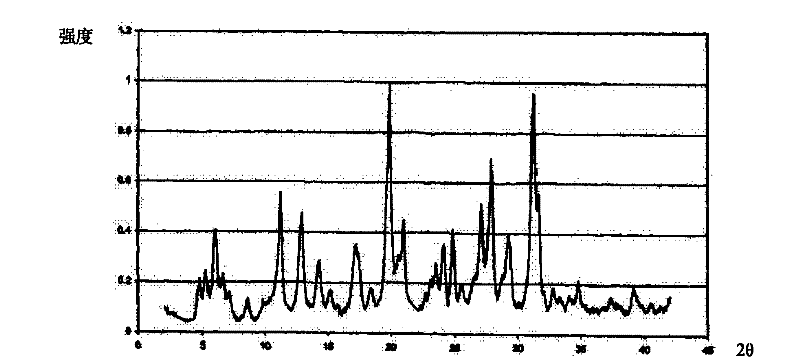
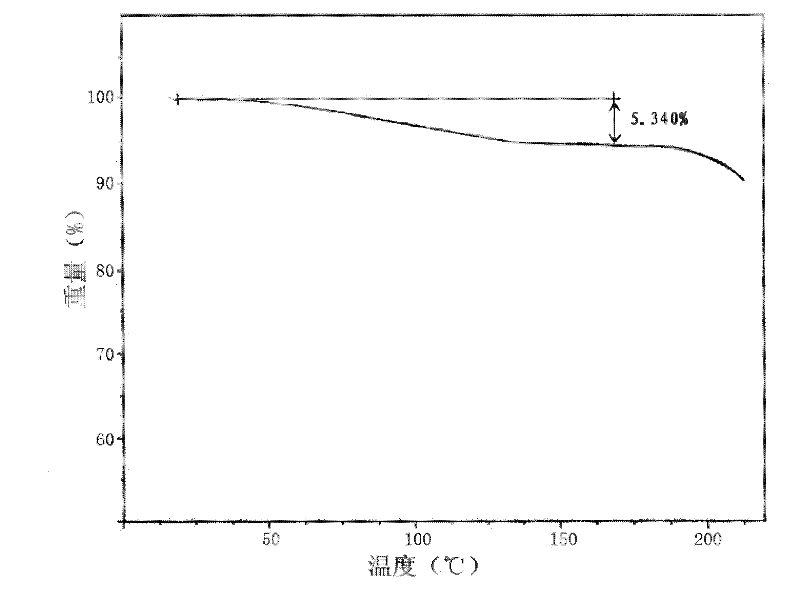
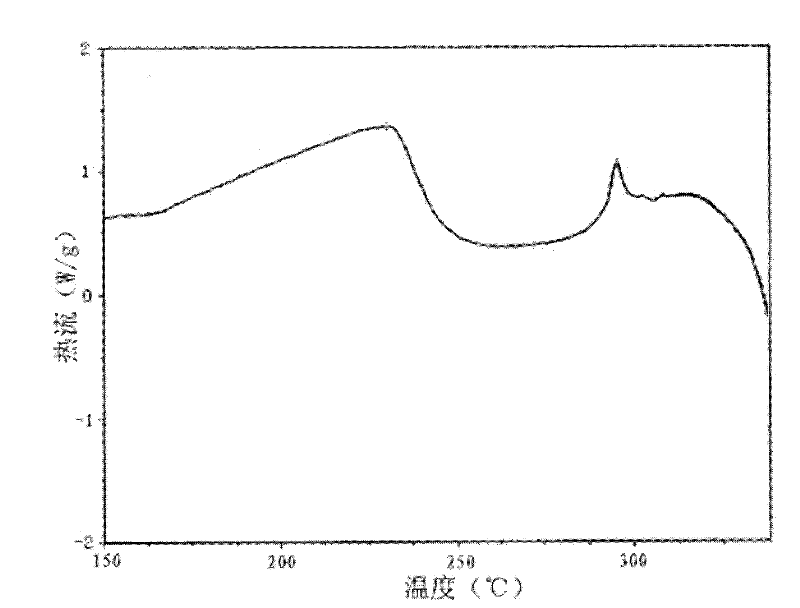
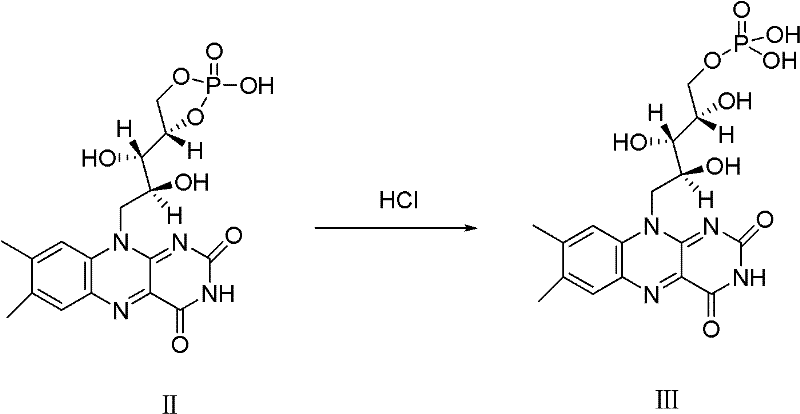
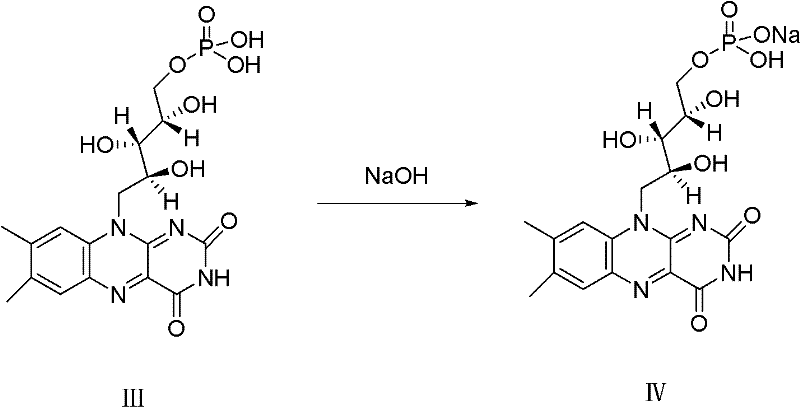
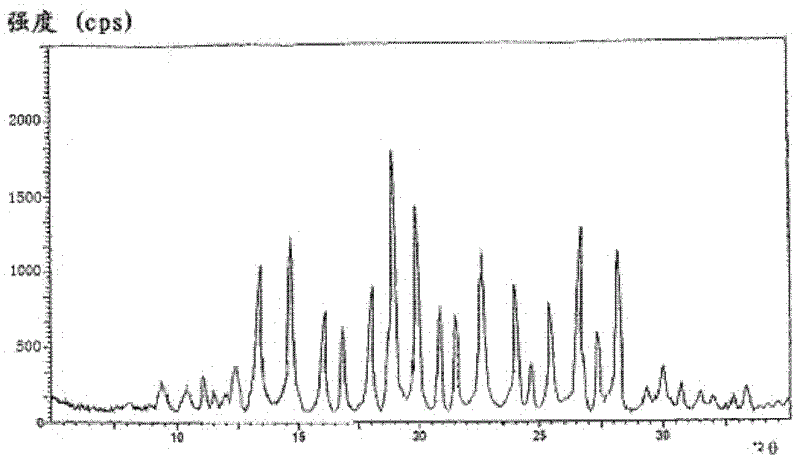
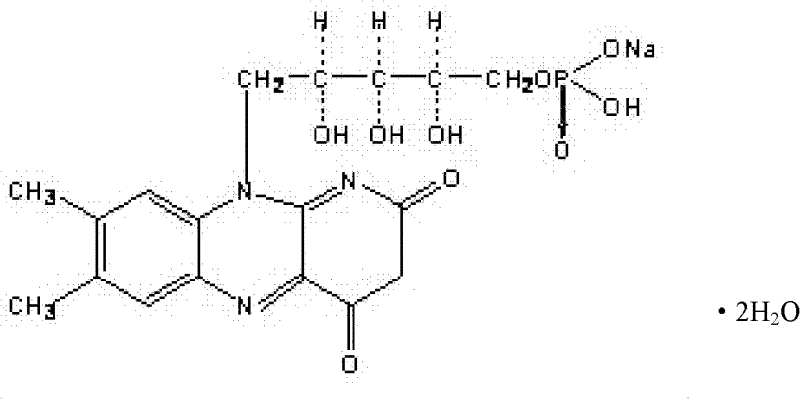
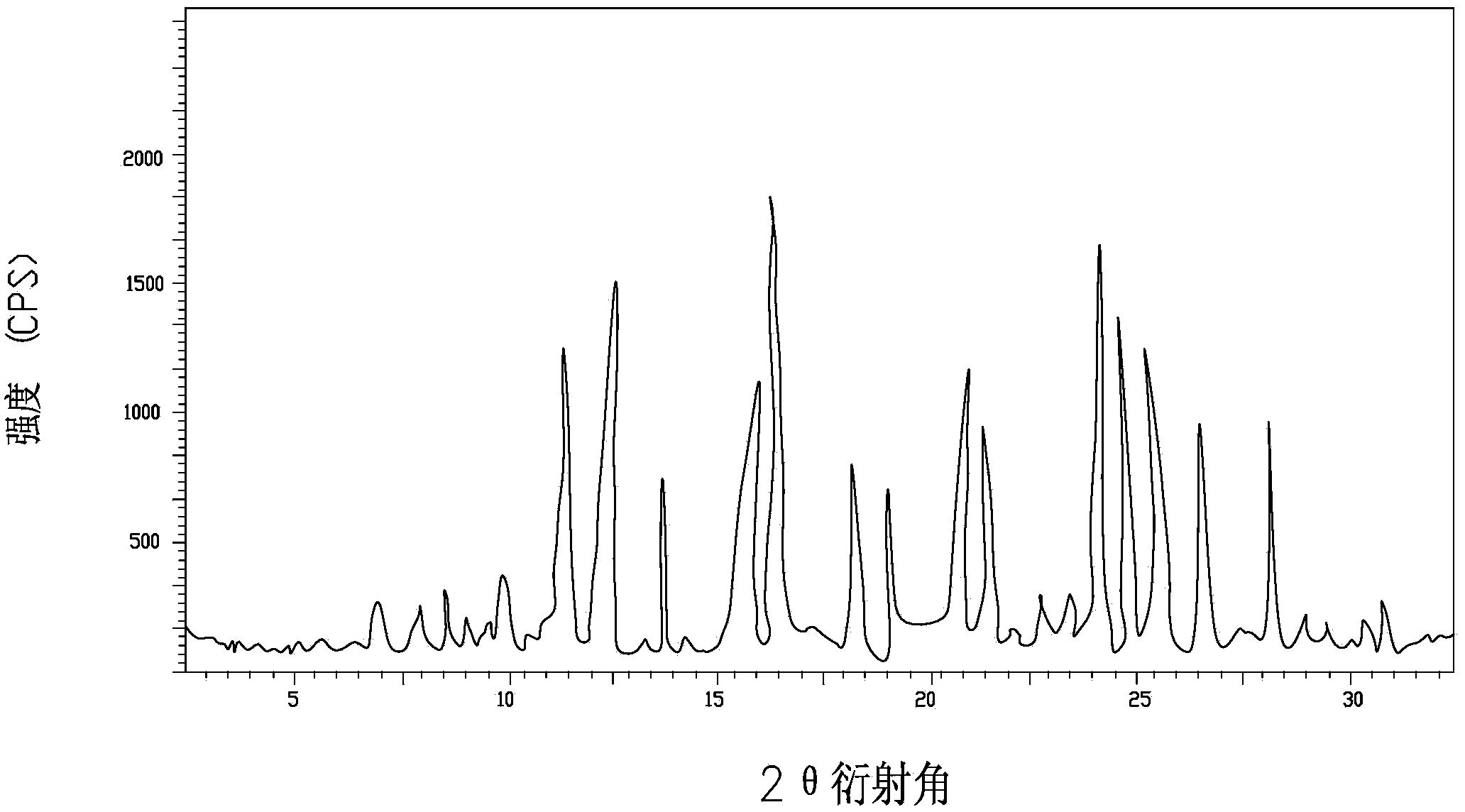
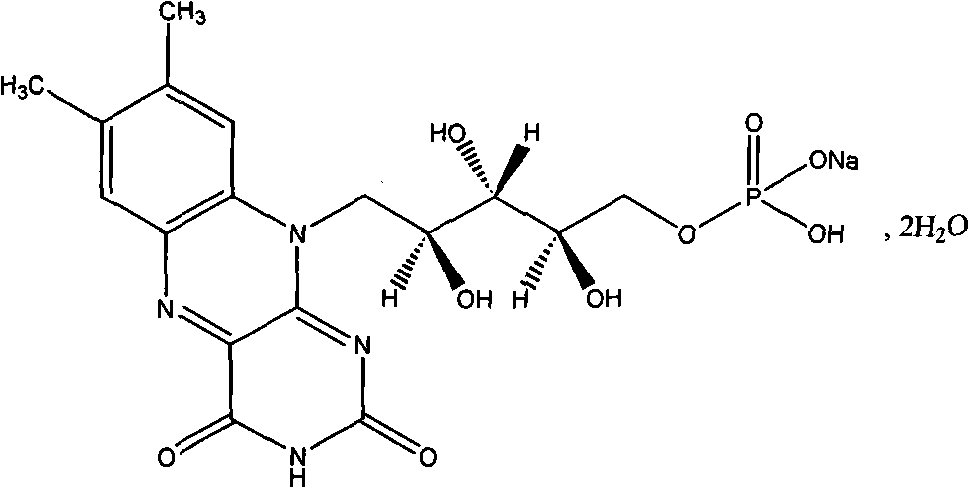
A riboflavin sodium phosphate crystal compound, its pharmaceutical composition and preparation method
ActiveCN102260291AHigh puritySimple processOrganic active ingredientsMetabolism disorderPowder diffractionCrystallization
The invention relates to a riboflavin sodium phosphate crystalline compound, which comprises 1.5 crystal waters. The riboflavin sodium phosphate crystalline compound is measure by using Cu-Kalpha rays. Characteristic peaks are shown when X-ray powder is diffracted at 2theta of 6.1 DEG, 11.2 DEG, 13.4 DEG, 14.8 DEG, 19.7 DEG, 20.8 DEG, 23.9 DEG, 24.5 DEG, 26.9 DEG, 27.7 DEG, 29.1 DEG, 31.0 DEG, and 31.6 DEG. The invention also relates to a preparation method of the compound, and a pharmaceutical composition containing the compound. The invention further relates to a synthesizing method of riboflavin sodium phosphate. As a result of experiments, the riboflavin sodium phosphate crystalline compound provided by the invention has high stability.
Owner:周晓东
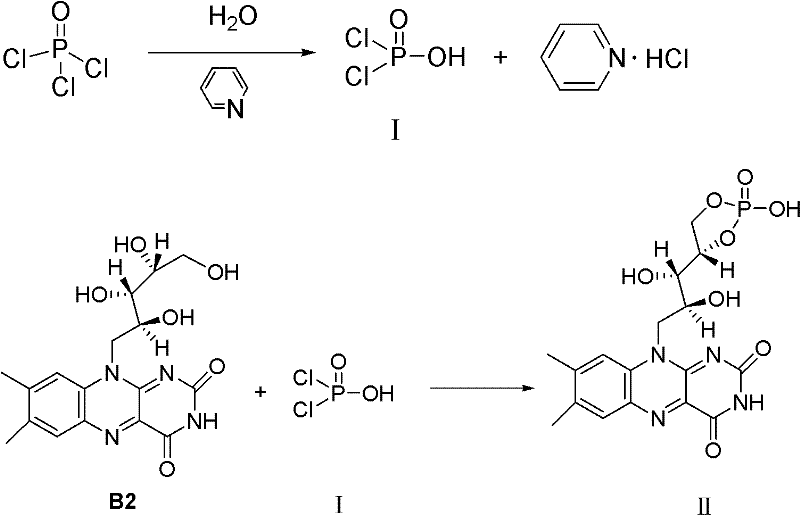
Industrial preparation method for riboflavine sodium phosphate
ActiveCN102206233AMild production conditionsReduce contentGroup 5/15 element organic compoundsRIBOFLAVIN PHOSPHATESodium phosphates
The invention discloses an industrial preparation method for riboflavin sodium phosphate, which uses riboflavin, phosphorus oxychloride, pyridine and purified water as initial raw materials, and comprises the following steps of: 1) carrying out phosphation reaction on riboflavin to generate riboflavin cyclophosphate; 2) hydrolyzing the riboflavin cyclophosphate to generate riboflavin phosphate; 3) carrying out salifying reaction on the riboflavin phosphate to generate riboflavin sodium phosphate; and 4) refining the riboflavin sodium phosphate. The riboflavin sodium phosphate product prepared by the preparation method provided by the invention has high quality and low impurity content, and can reach the quality requirement on riboflavin sodium phosphate in various current pharmacopoeias at home and abroad. The preparation method has the advantages of mild production conditions, no requirements for very low temperature, extra-high temperature and extra-high pressure, no special requirement on equipment, easiness in mastery of production technique, low production cost of a product, high product purity and easiness in recycling of wastes, and is suitable for industrial mass production.
Owner:HUBEI GUANGJI PHARMA
Production technology for riboflavin sodium phosphate
InactiveCN107286194AReduce processingLow costOrganic chemistry methodsGroup 5/15 element organic compoundsPhosphorylationHydrolysis
The invention relates to a production technology for riboflavin sodium phosphate. The invention aims to realize simple technology, easy control and high yield. The production technology comprises the following steps: performing phosphorylation reaction: adding phosphorus oxychloride and acetonitrile in turn, dropwise adding pyridine of which the weight is 2 / 3 of the total weight, slowly dropwise adding a solution prepared from the residual pyridine and purified water and reacting for 30-60 minutes; adding vitamin B2 for performing phosphorylation reaction for 20-40 minutes under the temperature at 30-37 DEG C; while crystallizing, adding riboflavin cyclophosphate seed crystal of which the weight is 0.25-0.5% of the weight of the vitamin B2 for promoting the crystallization; performing hydrolysis reaction: adding 18%-20% hydrochloric acid solution into the separated riboflavin cyclophosphate crystal and performing hydrolysis reaction for 1-3 hours under the temperature at 45-55 DEG C; adding sodium hydroxide for performing neutral reaction and separating, thereby acquiring a riboflavin sodium phosphate crude product; washing with methyl alcohol and ethanol solution and removing the impurities and organic residual matters from the riboflavin sodium phosphate; drying and crushing to the required particle size.
Owner:山西集翔生物工程有限公司
Bacteriostat-free water-soluble vitamin freeze-dried preparation for injection
ActiveCN101007018AThoroughly sterilizedUniform and thorough continuous sterilizationOrganic active ingredientsPowder deliveryGlycineVitamin B12
Disclosed is an water soluble vitamin freeze-drying preparation containing no bacteria inhibitor, wherein each bottle of the preparation comprises constituents of aneurine mononitrate 2.79-3.41mg, riboflavin sodium phosphate 4.41-5.39mg, nicotinamide 36-44mg, chlorhydric pyridoxine 4.41-5.39mg, sodium pantothenate 14.85-18.15mg, vitamin C sodium 101.7-124.3mg, biotin 54-66mug, folic acid 0.36-0.44mg, vitamin B12 4.5-5.5mug, glycine 270-330mg, and dium ethylenediamine tetraacetate 0.45-0.55mg.
Owner:费森尤斯卡比华瑞制药有限公司
Composite vitamin injection pharmaceutical composition containing 13 vitamins and preparation method thereof
ActiveCN104415041ASolve instabilityImprove product qualityHydroxy compound active ingredientsMetabolism disorderVitamin injectionAntioxidant
The invention provides a composite vitamin injection pharmaceutical composition containing 13 vitamins and a preparation method thereof. The composition comprises 4 fat-soluble vitamins and 9 water-soluble vitamins, which are respectively placed in two ampoules, wherein the first ampoule contains vitamin A palmitate, vitamin K1, vitamin D3, vitamin E, vitamin C, vitamin B1, riboflavin sodium phosphate, dexpanthenol, vitamin B6, nicotinamide and a cosolvent, an antioxidant, and a pH adjusting agent; the second ampoule contains vitamin B12, folic acid, biotin, a stabilizing agent and a pH adjusting agent. The preparation method comprises that the preparation of the first ampoule comprises steps of oil-phase medicinal liquid preparation, water-phase medicinal liquid preparation, mixing, pH adjustment, filtration, filling, sterilization, and the like, and the preparation of the second ampoule comprises steps of medicinal liquid preparation, pH adjustment, active carbon adsorption, decarburization, filtration, filling, fusing sealing, sterilization and the like. According to the invention, the solubilizer, the antioxidant, and the preparation process of the composite vitamin injection pharmaceutical composition containing 13 vitamins are improved, and the composite vitamin injection pharmaceutical composition has the advantages of few using kinds of auxiliary materials, good stability, high clarity, high security, simple preparation method, and easy realization of industrialization.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Riboflavin sodium phosphate compound
ActiveCN102617643AControl granularityPromote formationGroup 5/15 element organic compoundsSolubilityWater soluble
The invention relates to a riboflavin sodium phosphate compound, which exists in a crystal form. X-ray powder diffraction measured by Cu-K alpha rays shows that characteristic peaks exist at 2Theta of 12.4 degrees, 13.3 degrees, 14.7 degrees, 16.1 degrees, 16.9 degrees, 18.1 degrees, 18.9 degrees, 19.9 degrees, 20.9 degrees, 21.5 degrees, 22.7 degrees, 24.0 degrees, 24.7 degrees, 25.4 degrees, 26.7 degrees, 27.4 degrees and 28.2 degrees. The riboflavin sodium phosphate compound has main particle sizes of 5 to 15 mu m, preferably 6 to 12 mu m, and the distribution width of 2 to 20 mu m, preferably 3 to 18 mu m. A stability experiment shows that the riboflavin sodium phosphate crystal compound has high stability and better water solubility.
Owner:珠海晨安医药有限公司
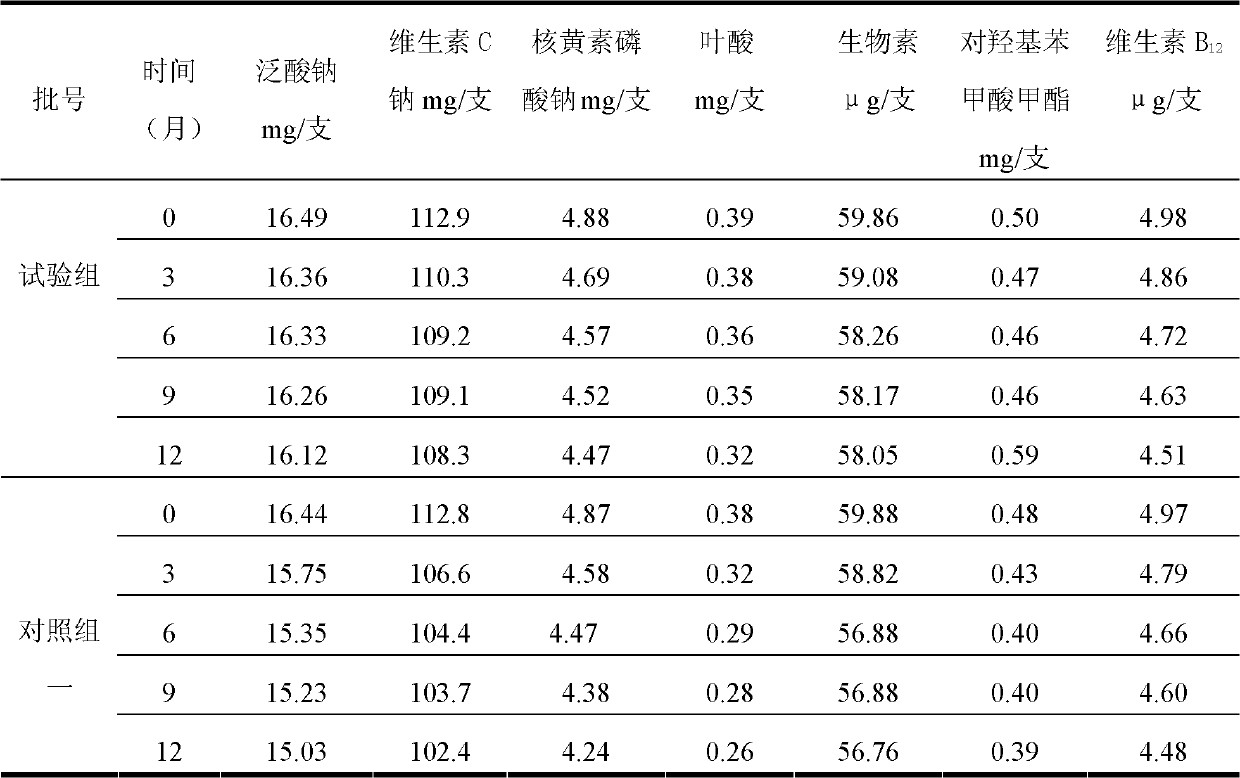
Water-soluble vitamin freeze-dried preparation for injection and preparation method thereof
ActiveCN103110656AImprove stabilityGuarantee drug safetyPowder deliveryMetabolism disorderFreeze-dryingSodium ascorbate
The invention discloses a water-soluble vitamin freeze-dried preparation for injection. 1000 bottles of freeze-dried preparations are prepared from the following raw materials: 2.8-3.4g of thiamine mononitrate, 36-44g of nicotinamide, 4.4-5.4g of pyridoxine hydrochloride, 14.8-18.1g of sodium pantothenate compound, 4.4-5.4g of riboflavin sodium phosphate, 102-124g of sodium ascorbate, 54-66mg of biotin, 0.36-0.44g of folic acid, 4.5-6.0mg of vitamin B12, 0.4-0.6g of methyl p-hydroxybenzoate, 280-320g of glycine, 0.4-0.6g of ethylene diamine tetraacetic acid disodium salt and 2000-3000ml of water for injection. The stability of existing sodium pantothenate is obviously improved by the obtained sodium pantothenate compound, thus ensuring that the sodium pantothenate containing water-soluble vitamin freeze-dried preparation for injection has ideal stability and curative effect and further ensuring medication safety of the patients.
Owner:SHANXI PUDE PHARMA CO LTD
Multivitamin composition and preparation method thereof
The invention relates to a multivitamin composition and a preparation method thereof, the multivitamin composition contains vitamin A palmitate, vitamin D3, vitamin E, vitamin K1, vitamin C, vitamin B1, riboflavin sodium phosphate, vitamin B6, vitamin B12, folic acid, dexpanthenol, biotin, nicotinamide, histidine and its salts and polysorbate 80, and compared with the prior art, the multivitamin composition has better solution clarity and can better ensure drug safety and convenient clinical use.
Owner:北京藏卫信康医药研发有限公司
Preparation method of lithium iron phosphate/graphene composite material
InactiveCN107154489ALower resistanceExcellent rate performanceCell electrodesCarbon compositesHigh rate
The invention discloses a preparation method of a lithium iron phosphate / graphene composite material. The preparation method comprises the following steps of adding graphene and riboflavin sodium phosphate into a solvent; treating by ultrasonic waves, stirring, and uniformly dispersing; respectively dissolving a complexing agent, a lithium source compound and an iron salt compound into a solution, then sequentially adding into a graphene dispersing liquid, and reacting, so as to obtain the lithium iron phosphate / graphene composite material; uniformly mixing the obtained lithium iron phosphate / graphene composite material and a carbon source in a ball milling way, drying, and calcining for 3 to 24h at constant temperature of 600 to 850 DEG C under the inert gas protection atmosphere, so as to obtain the graphene-modified lithium iron phosphate / carbon composite material. The preparation method has the advantage that the riboflavin sodium phosphate is dispersed into the graphene to form a surfactant, and can also be used as a phosphor source in the generation process of the lithium iron phosphate. The prepared graphene-modified lithium iron phosphate / carbon composite material can be used as the cathode material of a lithium ion battery, so that the electrochemical properties, particularly the cyclic stability at high rate, of the battery can be obviously improved.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
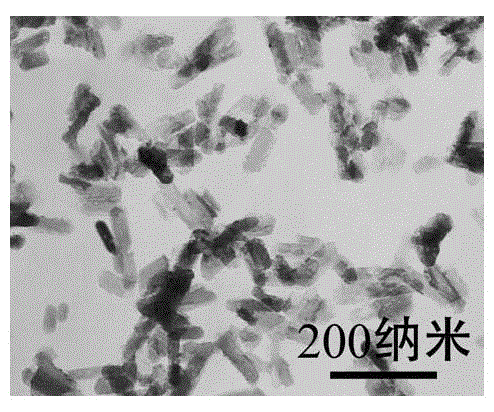
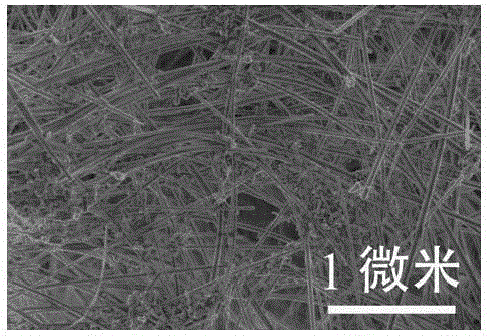
Method for preparation of hydroxyapatite nanorod and nanowire by hydrothermal process
ActiveCN103407979BSimple processLow costMaterial nanotechnologyPhosphorus compoundsSaline waterNanowire
The invention relates to a method for preparation of a hydroxyapatite nanorod and a nanowire by a hydrothermal process. The method includes: adding a water soluble calcium salt solution into a riboflavin sodium phosphate solution at a rate of 3-50ml / min, adjusting the pH of the obtained mixed solution to 8-12 or 4-6, then leaving the solution to undergo a hydrothermal reaction at 100-200DEG C for 1-48h, carrying out separation, washing and drying the precipitate, thus obtaining the hydroxyapatite nanorod and nanowire.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Compound vitamin medicine composition for injection and preparation method of compound vitamin medicine composition
InactiveCN104606209AImprove stabilityStable pHPowder deliveryMetabolism disorderHydrogenBULK ACTIVE INGREDIENT
The invention discloses a compound vitamin medicine composition for injection and a preparation method of the compound vitamin medicine composition and belongs to the field of pharmacy. The compound vitamin medicine composition for injection in unit preparation comprises the following active ingredients: 110 mg of vitamin B, 5 mg of riboflavin sodium phosphate calculated by riboflavin and 200 mg of vitamin C. The composition has the advantages that pH (potential of hydrogen) is stable, the degradation of vitamin C is reduced, the stability of the active ingredients is improved, a preparation technology is simple, the production time is shortened, the safety is high, and the composition is suitable for mass production.
Owner:王大光
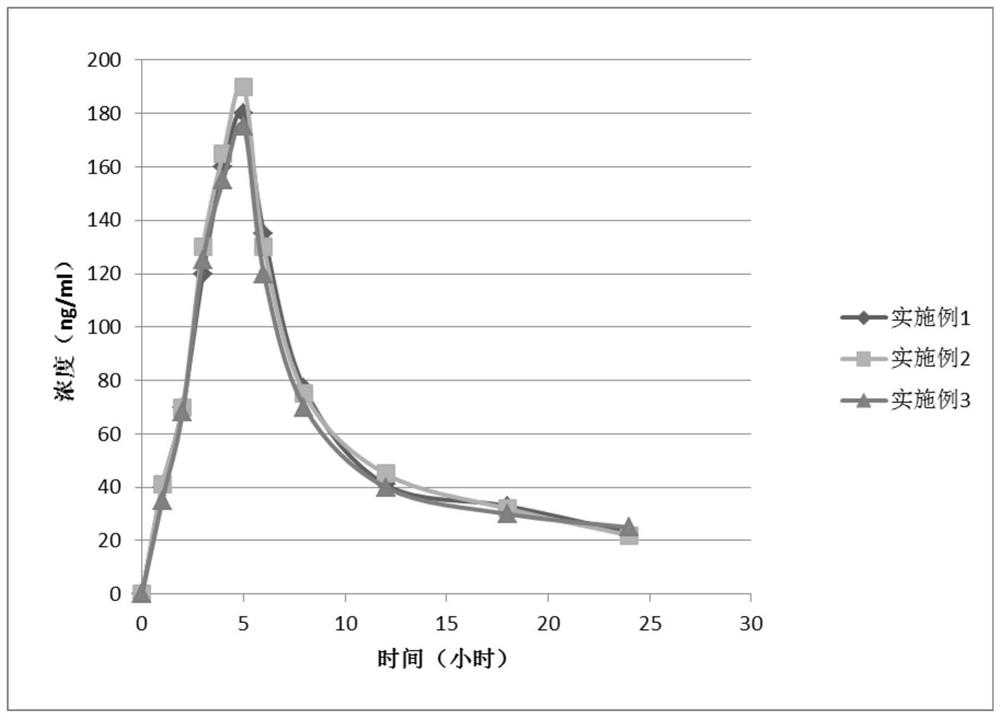
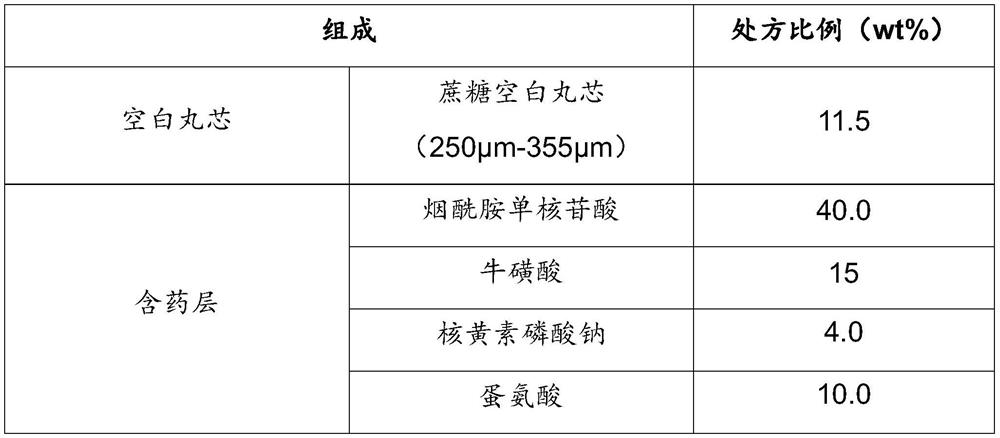
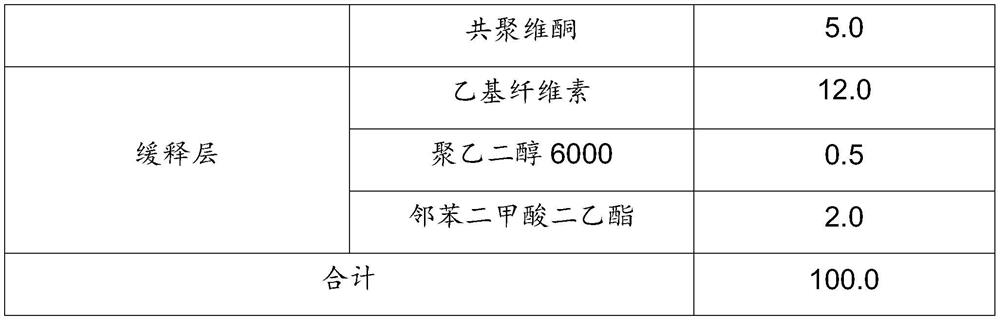
Nicotinamide mononucleotide sustained-release pellets and preparation method thereof
PendingCN112089706AAbility to promote oxidative phosphorylationIncreased lipid oxidationNervous disorderAntinoxious agentsSustained release pelletsSodium phosphates
The invention relates to nicotinamide mononucleotide sustained-release pellets and a preparation method thereof, and belongs to the field of medicine / health-care product preparations. The sustained-release pellets contain nicotinamide mononucleotide, taurine, riboflavin sodium phosphate, methionine, an adhesive and other substances, can safely, stably and permanently exert anti-aging and anti-oxidation effects, and also can promote metabolism of nicotinamide accumulated in the body, and multiple components cooperate with one another and are more effective than single use. The method is simpleto operate, low in cost and suitable for industrial production.
Owner:深圳深刻实验室有限公司
Riboflavin sodium phosphate compound and preparation method thereof
ActiveCN104072540AGood water solubilityHigh purityGroup 5/15 element organic compoundsSolubilityX-ray
The invention discloses a riboflavin sodium phosphate compound. The riboflavin sodium phosphate is measured by using powder X-ray diffractometry, and characteristic diffraction peaks are displayed at positions of 11.87 degrees, 12.56 degrees, 13.90 degrees, 16.22 degrees, 16.98 degrees, 18.13 degrees, 19.02 degrees, 20.92 degrees, 21.54 degrees, 24.17 degrees, 24.89 degrees, 25.38 degrees, 26.78 degrees and 28.45 degrees in an X-ray powder diffraction pattern expressed by a 2theta diffraction angle. The invention also discloses a method for preparing the riboflavin sodium phosphate compound as well as freeze-dried powder injection, hydro-acupuncture or sterile powder injection prepared by utilizing the riboflavin sodium phosphate compound. The riboflavin sodium phosphate compound prepared by the invention has the advantages of stable property, oxidation difficulty and high water solubility.
Owner:深圳朗欧医药集团有限公司
Water-soluble vitamin combination medicament
InactiveCN103656611APyrogenic reaction noEliminate reactivityMetabolism disorderDigestive systemSodium pantothenatePharmacology
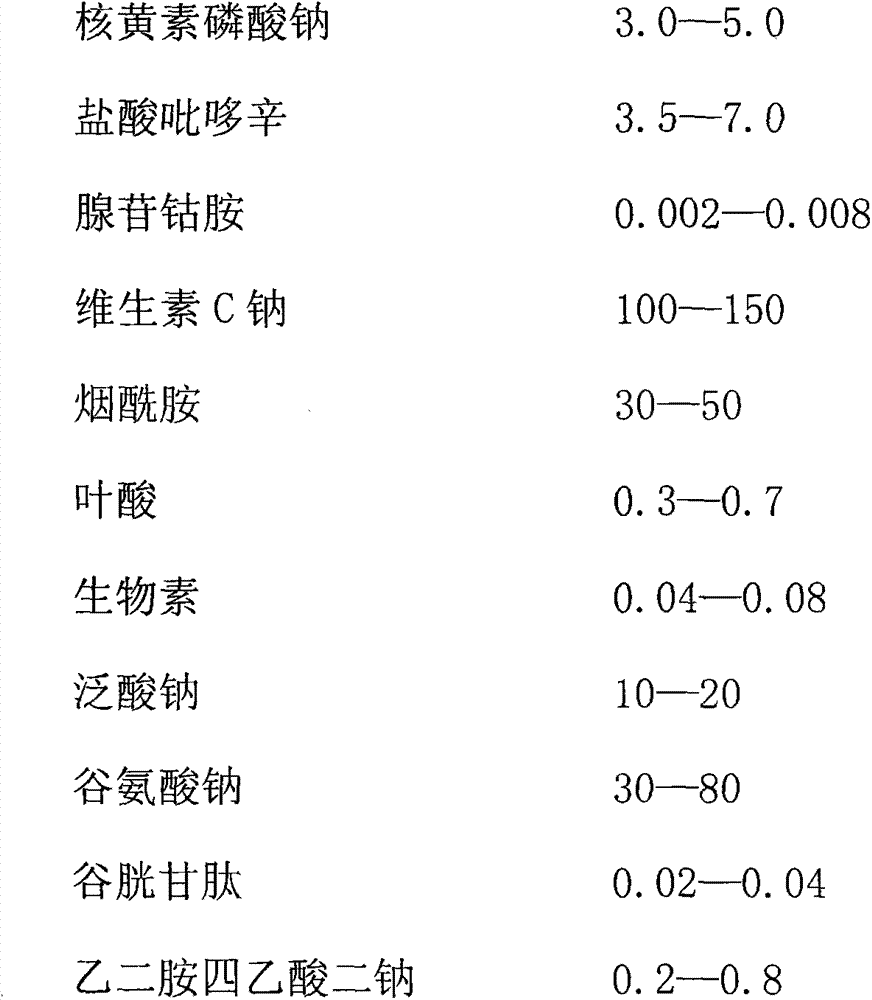
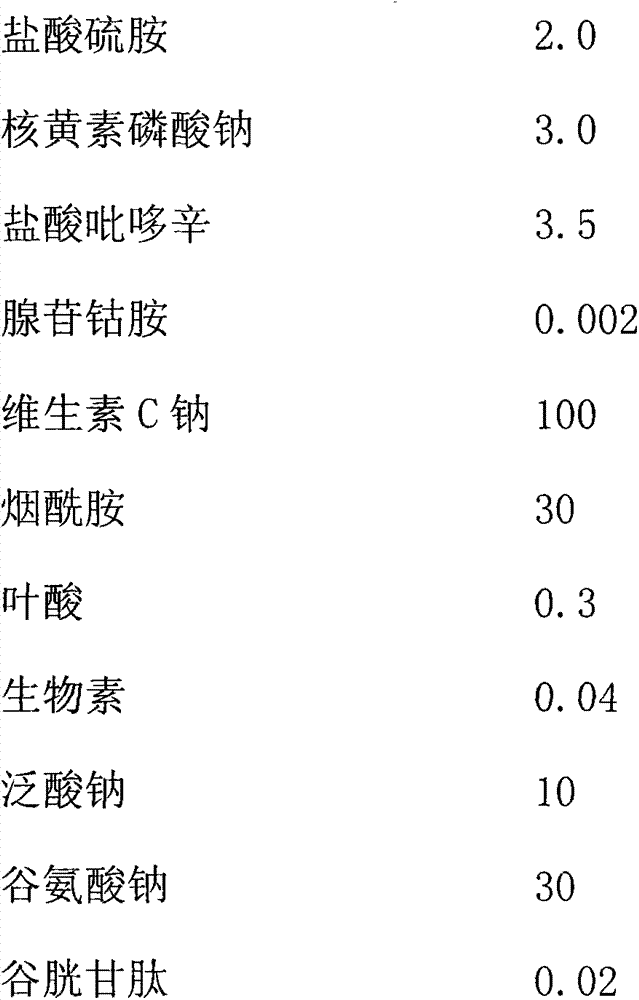
The invention provides a water-soluble vitamin combination medicament which is characterized by being prepared from the medical components in parts by weight: 2.0-4.0 parts of thiamine hydrochloride, 3.0-5.0 parts of riboflavin sodium phosphate, 3.5-7.0 parts of pyridoxine hydrochloride, 0.002-0.008 part of cobamamide, 100-150 parts of vitamin C sodium, 30-50 parts of nicotinamide, 0.3-0.7 part of folic acid, 0.04-0.08 part of biotin, 10-20 parts of sodium pantothenate, 30-80 parts of sodium glutamate, 0.02-0.04 part of reduced glutathione and 0.2-0.8 part ethylenediamine tetraacetic acid disodium. The invention also provides a preparation method for the combination medicament. All the aspects of safety, stability and treating effect of the water-soluble vitamin combination medicament disclosed by the invention are higher than those of the conventional water-soluble vitamin medicament prepared in the prior art.
Owner:吴赣英
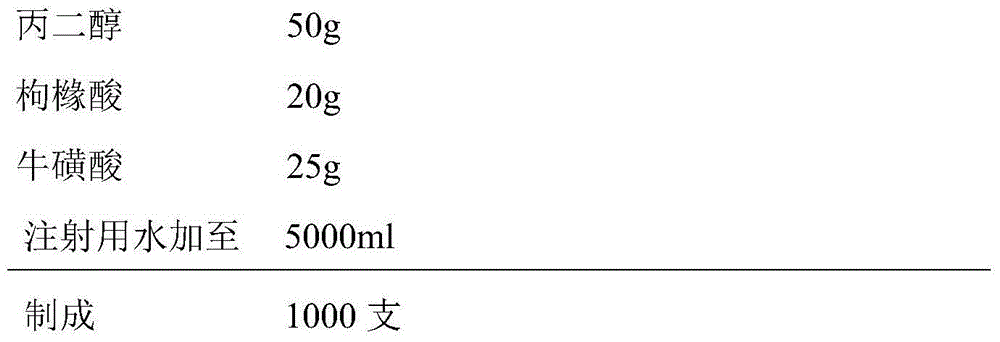
Compound vitamin freeze-dried powder injection for injection
The invention provides a compound vitamin freeze-dried powder injection for injection, which comprises vitamin B15-15 parts by weight, riboflavin sodium phosphate 3-8 parts by weight (counting by reducing crystal water), vitamin C 150-250 parts by weight, glycine 350-500 parts by weight and water for injection 1000-5000 parts by weight which is used as a solvent during preparation and is finally removed. The compound vitamin freeze-dried powder injection for injection is good in stability, high in safety and convenient to store and transport. The invention further provides a preparation method of the compound vitamin freeze-dried powder injection for injection. The compound vitamin freeze-dried powder injection for injection prepared through the preparation method is good in stability and high in safety. The invention further provides an analysis method of the compound vitamin freeze-dried powder injection for injection, and the analysis method is particularly specific to relevant substance of unstable basic medicines.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
A composite vitamin injection for veterinary use and a preparing method thereof
InactiveCN105748488ASignificant effectStable in natureMetabolism disorderPharmaceutical delivery mechanismVitamin b6Vitamin E Acetate
A composite vitamin injection for veterinary use and a preparing method thereof are disclosed. The injection comprises vitamin A palmitate, vitamin D3, vitamin E acetate, vitamin B1, riboflavin sodium phosphate, vitamin B6, nicotinamide, dexpanthenol, vitamin B12, Tween-80, disodium ethylenediamintetraacetate, ethylparaben, butylparaben, and the like. By utilizing the Tween-80 and other auxiliary agents, lipid-soluble vitamins are dissolved into water to prepare the composite vitamin injection comprising the lipid-soluble vitamins and water-soluble vitamins. The injection has significant curative effects on malnourished livestock. Properties of a product of the injection are stable. In addition, the injection is a composite injection, is convenient to use, and achieves treating effects of a plurality of medicines through one time of administration.
Owner:CHONGQING BULL ANIMAL PHARMA
Compound vitamin injection medicine composition and preparation method
ActiveCN104523714AStable pHImprove stabilityMetabolism disorderPharmaceutical non-active ingredientsVitamin injectionBULK ACTIVE INGREDIENT
The invention discloses a compound vitamin injection medicine composition and a preparation method, and belongs to the field of medicine manufacturing. According to the compound vitamin injection medicine composition, the active ingredients per dosage unit comprise 110 mg of vitamin B, 5 mg of riboflavin in riboflavin sodium phosphate and 200 mg of vitamin C. The compound vitamin injection medicine composition and the preparation method have the advantages that the PH is stable, the PH value is small in change, the content of main drugs is stable, related matter is less, the process is simplified, the liquid dosing time is shortened, and the operation difficulty is lowered.
Owner:北京柏雅联合药物研究所有限公司
Hydrophilicity modification method of hydroxyapatite single-crystal nanorod
InactiveCN105133022ALow costThe synthesis process is simplePolycrystalline material growthFrom normal temperature solutionsCarbamateSodium phosphates
The invention discloses a hydrophilicity modification method of a hydroxyapatite single-crystal nanorod. The hydrophilicity modification method comprises the following steps: firstly, synthesizing hydroxyapatite nanocrystalline with the surface modified by oleic acid; carrying out reflux reaction on the hydroxyapatite nanocrystalline with the surface modified by oleic acid and adenosine 5'-monophosphate sodium salt or riboflavin sodium phosphate, according to the mass ratio of 4: 1, in an acetone solvent for surface ligand exchange, so as to prepare a sample A; reacting the sample A with S-isocyanic acid-N-ethyl-N'-phenyl dithiocarbamate, so as to obtain a hydroxyapatite nanocrystalline sample B with the surface modified by a chain transfer agent; carrying out reversible addition-fragmentation chain transfer polymerization reaction on the sample B and a hydrophilic polymer. The hydrophilic nano-hydroxyapatite prepared through the method can be used in medicine carrying and substituting and cell imaging, can be used as an inorganic raw material for preparing degradable biomedical materials, such as artificial bones and bone cement, and has a good application prospect in relevant fields, such as biological materials and tissue engineering.
Owner:NORTHWEST UNIV
Compound vitamin lyophilized powder injection composition for injection and preparation method thereof
ActiveCN104490902ASmall fluctuationReduces multiple pH adjustment stepsPowder deliveryMetabolism disorderBULK ACTIVE INGREDIENTActive ingredient
The invention discloses a compound vitamin lyophilized powder injection composition for injection and a preparation method thereof, and belongs to the field of pharmacy. Unit preparation is prepared from 10mg of vitamin B1 serving as an active ingredient, 5mg of riboflavin sodium phosphate based on riboflavin and 200mg of vitamin C. The composition has the advantages of simple preparation process, short production time and high production efficiency, and the prepared product has controllable quality and excellent stability.
Owner:北京柏雅联合药物研究所有限公司
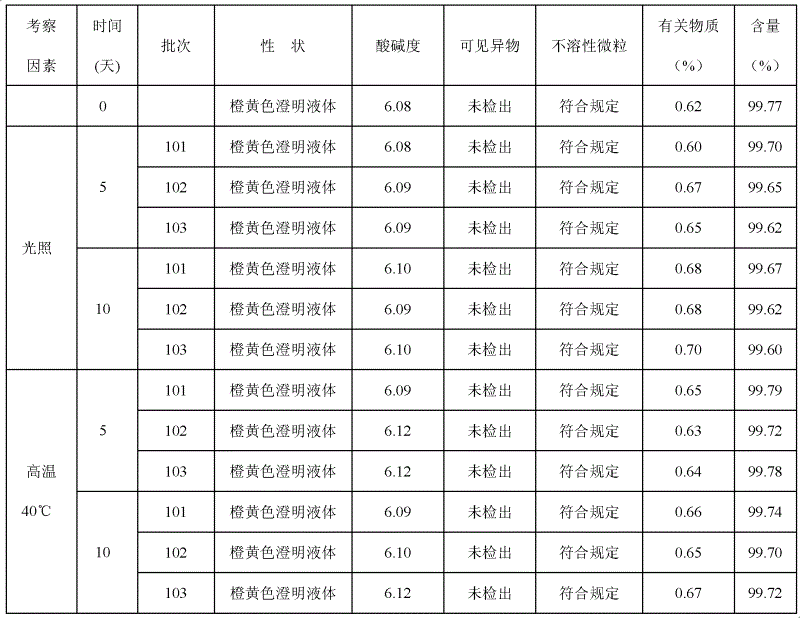
Riboflavin sodium phosphate freeze-dried powder injection and preparation method thereof
ActiveCN101874787AMeet the requirements for intravenous drug useReduce the risk of adverse reactionsOrganic active ingredientsPowder deliveryHaemolysisFreeze-drying
The invention relates to a riboflavin sodium phosphate freeze-dried powder injection and a preparation method thereof. The injection is characterized by comprising a riboflavin sodium phosphate dehydrate, mannitol and / or ammonia water. The injection is sensitive to light, so a brown amoxicillin bottle is used as the inner package of the injection. The riboflavin sodium phosphate freeze-dried powder injection has the following advantages: (1) single and clear auxiliary materials in the formula can meet the requirement of industrialized batch production; (2) medicinal injection grade raw and auxiliary materials accord with the medication requirement of human intravenous injection, so that potential adverse reaction risk in clinical application can be greatly reduced; (3) long-term stability tests prove that the preparation can be preserved for 2 years at the temperature below 30 DEG C, so that stability of the medicament in circulation and safety of clinical medication can be ensured; and (4) special safety tests, such as irritability, haemolysis, vascular stimulation and the like, prove that haemolysis, agglutination, stimulation and anaphylaxis reactions are not found.
Owner:HAINAN LEVTEC PHARMA
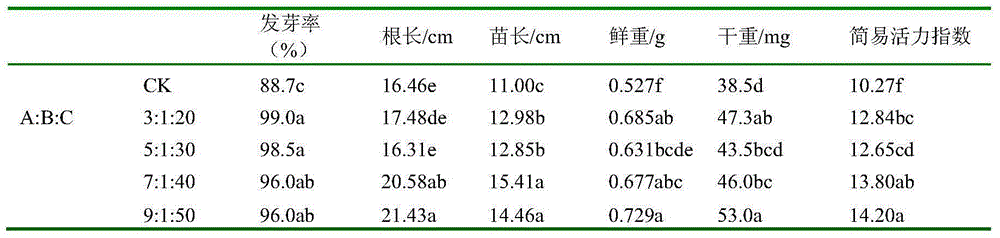
Seed treatment method for improving and enhancing vigor of sweet corn seeds and special compounding initiator for seed treatment method
ActiveCN105104415AQuality improvementIncrease costBiocidePlant growth regulatorsAcid waterSalicylic acid
The invention provides a seed treatment method for improving and enhancing the vigor of sweet corn seeds and a special compounding initiator for the seed treatment method. The compounding initiator is a mixture formed by a riboflavin sodium phosphate water solution with the concentration being 0.01% (w / v), a sodium succinate dibasic water solution with the concentration being 0.5 g / L and an acetylsalicylic acid water solution with the concentration being 0.5 mmol / L according to the volume ratio of 3-9:1:20-50. The seed treatment method for improving and enhancing the vigor of the sweet corn seeds through the compounding initiator is also within the protection range of the invention. The compounding initiator prepared from riboflavin sodium phosphate, sodium succinate dibasic and acetylsalicylic acid at different proportions is designed and combined with sand priming to treat and enhance the sweet corn seeds, several commonly-used vigor indexes are measured, and finally the formula of the compounding initiator capable of remarkably improving and enhancing the vigor of the sweet corn seeds is obtained. In addition, the high cost of riboflavin single dosage in the prior art can be greatly reduced through compounding of the three reagents, and certain universality is achieved.
Owner:BEIJING ZHONGNONGXINXING AGRI TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com