Hydrophilicity modification method of hydroxyapatite single-crystal nanorod
A hydroxyapatite, single crystal nanotechnology, applied in the direction of single crystal growth, single crystal growth, chemical instruments and methods, etc., can solve problems such as hydrophilicity limitation, achieve stable product quality, easy process amplification, and good application Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
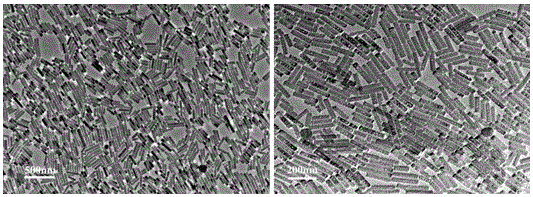
[0023] In a 100mL flask, add the above-mentioned 800mg hydrophobic hydroxyapatite nanorods, 200mg riboflavin sodium phosphate, and 50mL acetone solution, and reflux at 60°C for 6-8h to obtain sample A; The functional group (-OH) and the chain transfer agent CTA synthesize a new chain transfer agent, the reaction conditions: 600mg product A and 108mg S-isocyanate-N-ethyl-N'-phenyldithiocarbamate (CTA ) using N,N'-dicyclohexylcarbodiimide (DCC, 1.00g) as a dehydrating agent, 4-dimethylaminopyridine (DMAP, 200mg) as a catalyst to form a new chain transfer agent, and react at room temperature for 18-24h For sample B. Finally, 100mg of sample B was used as a new chain transfer agent, 12mg of azobisisobutyronitrile (AIBN) was used as a chain initiator, 1.00g of hydrophilic polymer polyethylene glycol methacrylate (PEGMA) was used as a monomer, and tetrahydrofuran was used as a monomer. solvent, reacted for 24h under nitrogen environment, and collected samples.
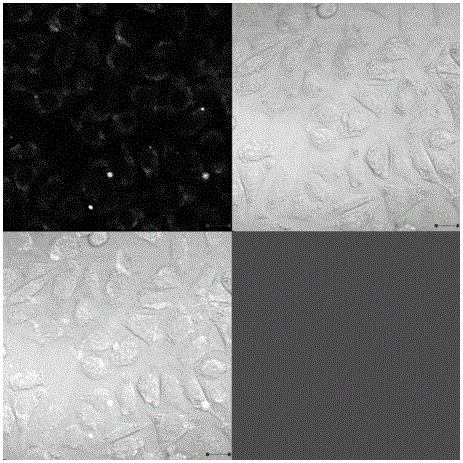
[0024] After modif...
Embodiment 2
[0026] In a 100mL flask, add 800mg hydrophobic hydroxyapatite nanorods, 200mg riboflavin sodium phosphate, 50mL acetone solution, and reflux at 60°C for 6-8h to obtain sample A; Functional group (-OH) and chain transfer agent CTA to synthesize a new chain transfer agent, reaction conditions: 600mg product A and 108mg S-isocyanate-N-ethyl-N'-phenyldithiocarbamate (CTA) Use N,N'-dicyclohexylcarbodiimide (DCC, 1.00g) as a dehydrating agent, and 4-dimethylaminopyridine (DMAP, 200mg) as a catalyst to form a new chain transfer agent. The reaction at room temperature for 18-24h is recorded as Sample B. Finally, 100mg of sample B was used as a new chain transfer agent, 12mg of azobisisobutyronitrile (AIBN) was used as a chain initiator, 2.00g of hydrophilic polymer polyethylene glycol methacrylate (PEGMA) was used as a monomer, and tetrahydrofuran was used as a monomer. solvent, reacted for 24h under nitrogen environment, and collected samples.
Embodiment 3
[0028] In a 100mL flask, add 800mg hydrophobic hydroxyapatite nanorods, 200mg riboflavin sodium phosphate, 50mL acetone solution, and reflux at 60°C for 6-8h to obtain sample A; Functional group (-OH) and chain transfer agent CTA to synthesize a new chain transfer agent, reaction conditions: 600mg product A and 108mg S-isocyanate-N-ethyl-N'-phenyldithiocarbamate (CTA) Use N,N'-dicyclohexylcarbodiimide (DCC, 1.00g) as a dehydrating agent, and 4-dimethylaminopyridine (DMAP, 200mg) as a catalyst to form a new chain transfer agent. The reaction at room temperature for 18-24h is recorded as Sample B. Finally, 100mg of sample B was used as a new chain transfer agent, 12mg of azobisisobutyronitrile (AIBN) was used as a chain initiator, 1.00g of hydrophilic polymer phospholipid was used as a monomer, tetrahydrofuran was used as a solvent, and the reaction was carried out under nitrogen for 24 hours to collect samples .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com