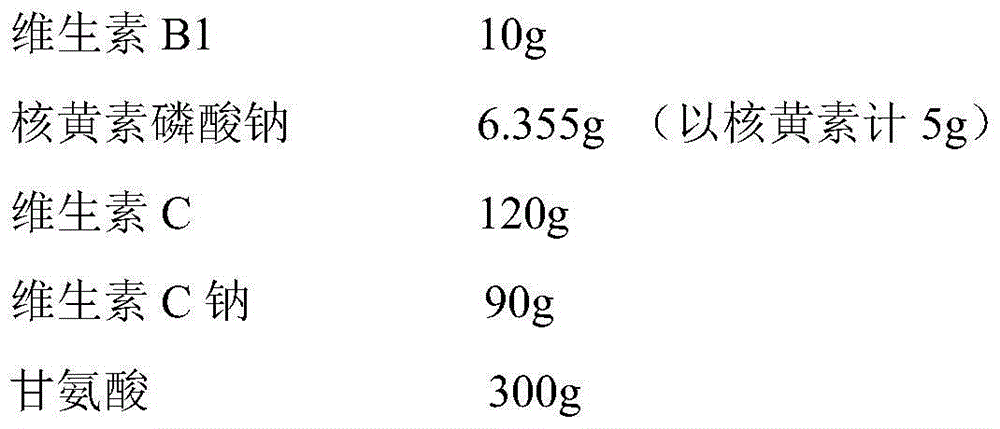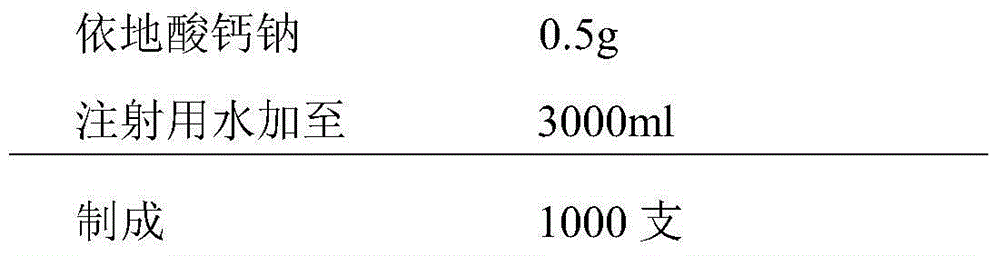Compound vitamin medicine composition for injection and preparation method of compound vitamin medicine composition
A technology for compound vitamins and vitamins, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, and freeze-dried delivery, etc., can solve the problems of large fluctuations in pH value and temperature, decline in product quality and stability, and accelerated decomposition of vitamin C, etc. Achieving the effect of less impurity content, avoiding degradation and impurity generation, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Prescription:
[0031]
[0032]
[0033] Two. Method
[0034] Weigh 120g vitamin C and 90g vitamin C sodium, add to 2500ml water for injection, stir to dissolve; add 300g glycine and 0.5g calcium sodium edetate, stir to dissolve, then add 10g vitamin B1 and 6.355g riboflavin Sodium phosphate (5 g in terms of riboflavin) was stirred and dissolved, and the pH value of the solution was about 4.0; adding activated carbon for needles with a weight to volume ratio of 0.05%, filtered after stirring for 30 minutes, and then added 500 ml of water for injection to 3000 ml; It is filtered through a microporous membrane, filled into 1000 preparation units, and freeze-dried to obtain the product.
[0035] The freeze-drying operation is as follows: the product is put into the freeze-drying box, pre-frozen to below -40°C, crystallized and heated to -12°C, then cooled to below -40°C, heated to 40°C after vacuuming, kept at this temperature for 8 hours, frozen dry end.
Embodiment 2
[0037] 1. Prescription:
[0038]
[0039] Two. Preparation method:
[0040] Weigh 63.8g vitamin C and 153.3g vitamin C sodium, add to 2700ml water for injection, stir to dissolve; add 300g glycine and 0.5g edetate calcium sodium, stir to dissolve, then add 10g vitamin B1 and 6.355g core Sodium flavin phosphate (5 g in terms of riboflavin) was stirred and dissolved; the pH value of the solution was about 4.5; adding gac for needles with a weight to volume ratio of 0.05%, filtered after stirring for 30 minutes, and then added 300 ml of water for injection to 3000 ml; Filter through a 0.22 μm microporous membrane, fill into 1000 preparation units, and freeze-dry to obtain the product.
[0041] The freeze-drying operation is as follows: the product is put into the freeze-drying box, pre-frozen to below -40°C, crystallized and heated to -12°C, then cooled to below -40°C, heated to 40°C after vacuuming, kept at this temperature for 8 hours, frozen dry end.
Embodiment 3
[0043] 1. Prescription:
[0044]
[0045] Two. Preparation method:
[0046] Weigh 25.7g vitamin C and 195.6g vitamin C sodium, add to 1800ml water for injection, stir to dissolve; add 300g glycine and 0.5g edetate calcium sodium, stir to dissolve, then add 10g vitamin B1 and 6.355g core Sodium flavin phosphate (5g in terms of riboflavin) was stirred and dissolved; the pH value of the solution was about 5.0; adding gac for needles with a weight to volume ratio of 0.05%, filtered after stirring for 30 minutes, and then added 1200ml water for injection to 3000ml; Filter through a 0.22 μm microporous membrane, fill into 1000 preparation units, and freeze-dry to obtain the product.
[0047] The freeze-drying operation is as follows: the product is put into the freeze-drying box, pre-frozen to below -40°C, crystallized and heated to -12°C, then cooled to below -40°C, heated to 40°C after vacuuming, kept at this temperature for 8 hours, frozen dry end.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com