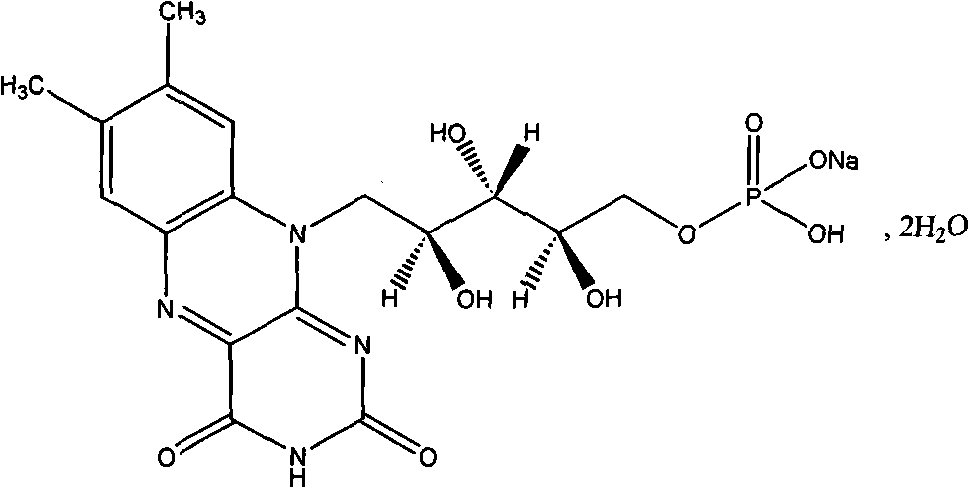
Riboflavin sodium phosphate freeze-dried powder injection and preparation method thereof
A riboflavin sodium phosphate, freeze-dried powder injection technology, applied in freeze-dried delivery, powder delivery, pharmaceutical formulations and other directions, can solve the problems of increasing unpredictable risks, easy turbidity of the solution, poor clarity, etc. The freeze-drying process is reasonable and feasible, the appearance is full, and the effect of reducing the risk of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Riboflavin sodium phosphate 5g (calculated as riboflavin)
[0022] Mannitol 50.0g
[0023] Ammonia amount
[0024] Add water for injection to 1000ml
[0025] Made 1000 pieces
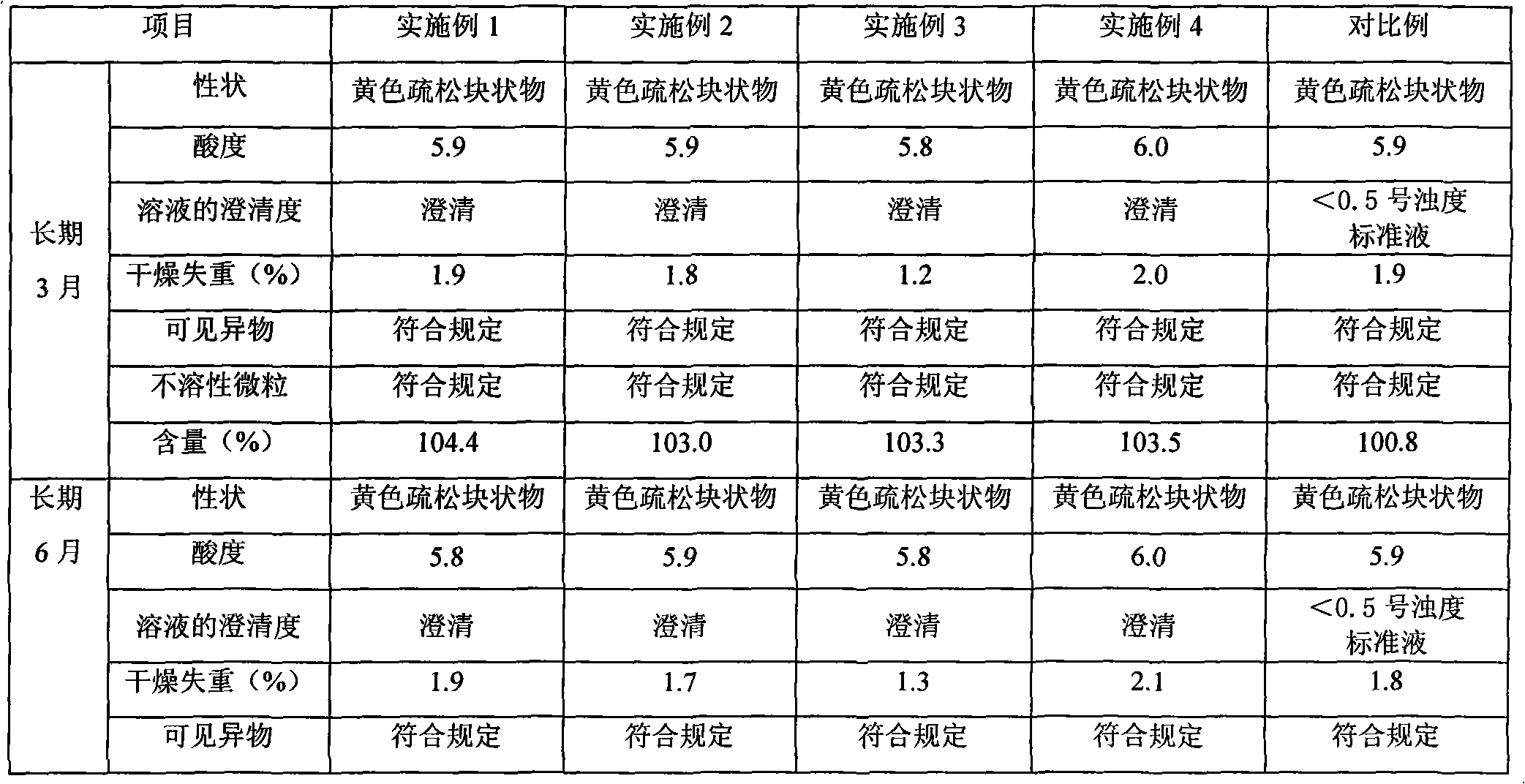
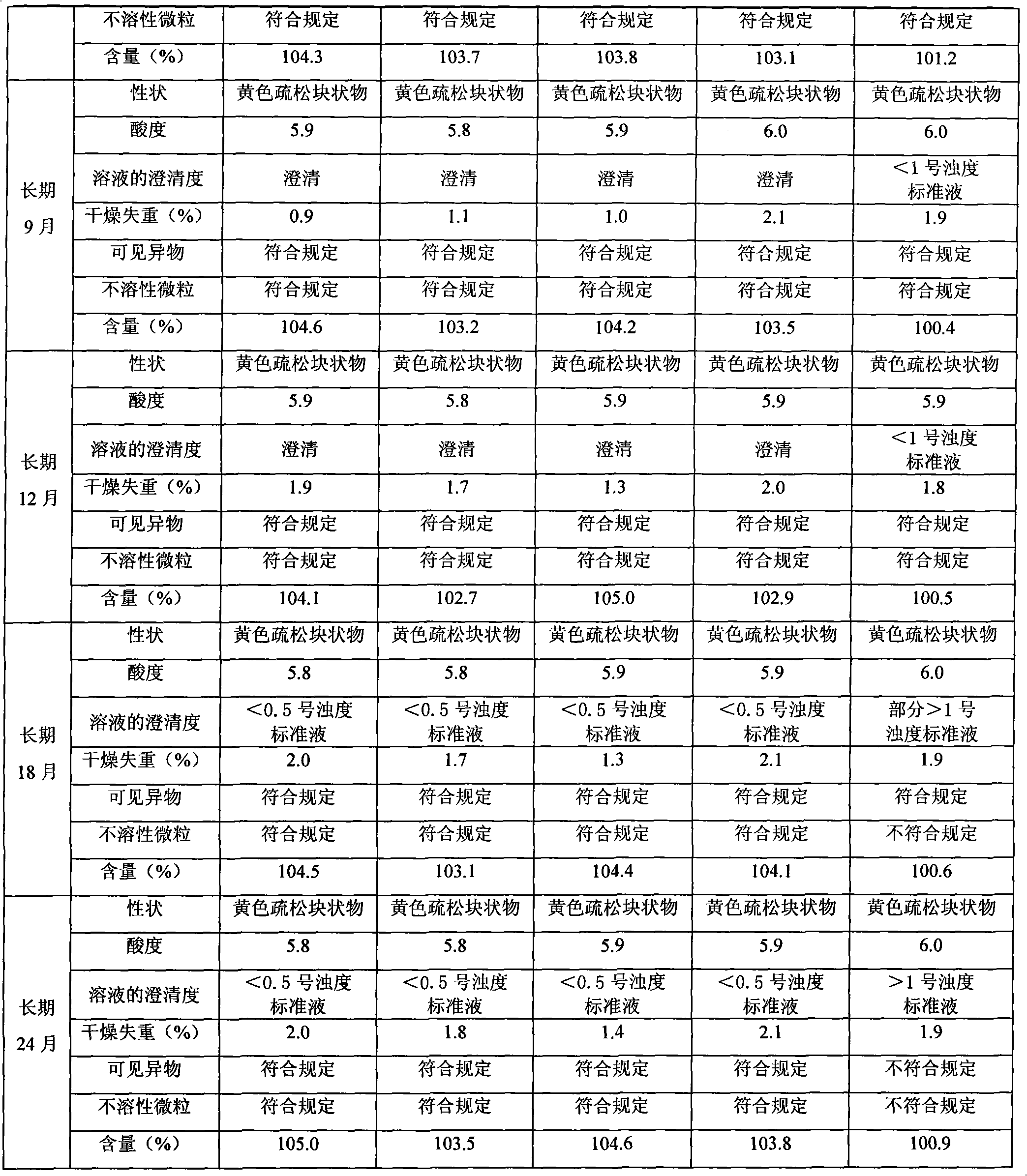
[0026] Preparation process: operate in the dark, weigh the prescribed amount of riboflavin sodium phosphate and mannitol, dissolve in an appropriate amount of water for injection, stir and dissolve, adjust the pH of the solution to 5.9 with ammonia solution, and add water for injection to the full amount; Then add activated carbon accounting for 0.1% (W / V) of the liquid amount, stir and decolorize for 20 minutes, recirculate and stir for 10 minutes, filter and decarbonize with a titanium filter stick, and then fine filter through a microporous membrane (0.22 μm membrane); The filtrate was tested for content, density, endotoxin, acidity, clarity, etc. After passing the test, it was filled in brown vials; the subpackaged samples were placed in a freeze-drying box, and -40°C was used as the pre-f...
Embodiment 2
[0028] Riboflavin sodium phosphate 10g (calculated as riboflavin)
[0029] Mannitol 100.0g
[0030] Ammonia amount
[0031] Add water for injection to 1000ml
[0032] Made 1000 pieces
[0033]Preparation process: operate in the dark, weigh the prescribed amount of riboflavin sodium phosphate and mannitol, dissolve in an appropriate amount of water for injection, stir and dissolve, adjust the pH of the solution to 6.0 with ammonia solution, and add water for injection to the full amount; Then add activated carbon accounting for 0.2% (W / V) of the liquid amount, stir and decolorize for 20 minutes, recirculate and stir for 10 minutes, filter and decarbonize with a titanium filter stick, and then finely filter through a microporous membrane (0.22 μm membrane); The filtrate was tested for content, density, endotoxin, acidity, clarity, etc. After passing the test, it was filled in brown vials; the subpackaged samples were placed in a freeze-drying box, and -40°C was used as the pr...
Embodiment 3
[0035] Riboflavin sodium phosphate 5g (calculated as riboflavin)
[0036] Mannitol 60.0g
[0037] Add water for injection to 1000ml
[0038] Made 1000 pieces
[0039] Preparation process: operate in the dark, weigh the prescribed amount of riboflavin sodium phosphate and mannitol and dissolve in an appropriate amount of water for injection, stir and dissolve, and measure the pH value of the solution to 5.9 (within the required range, the pH value of ammonia water is not used) , add water for injection to the full amount; then add activated carbon accounting for 0.1% (W / V) of the liquid amount, stir and decolorize for 20 minutes, recirculate and stir for 10 minutes, decarbonize with a titanium filter stick, and then pass through a microporous membrane (0.22 μm filter membrane) for fine filtration; take the filtrate for content, density, endotoxin, acidity, clarity and other tests, and fill it in a brown vial after passing the test; put the subpackaged sample into a freeze-dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com