Asarone drug composition for injection or inhalation
A technology of asarone and composition, which is applied in the field of asarone pharmaceutical composition, and can solve problems such as turbidity, decreased content, and poor resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2
[0054] Embodiment 1, 2 Preparation process: a. Weigh water for injection, put it into concentrated irrigation, add mannitol and polysorbate-80 in the prescribed amount, dissolve and stir evenly, add an appropriate amount of buffered saline solution to adjust the pH value of the solution Get solution I at 5.0~5.8; b, take the prescription amount of ethanol and polyethylene glycol 400, add asarone, stir to dissolve completely, and obtain solution II; c, add the prepared solution II to the total amount of the prescription in time In solution I, add water for injection to the full amount, add 0.1% activated carbon of the above total amount, stir and circulate for 30 minutes under nitrogen protection, and circulate and stir for 30 minutes through a microporous filter element to obtain an intermediate solution; d, take the intermediate solution for inspection , filled after meeting the requirements; e, freeze-dried, plugged, and out of the box to obtain the freeze-dried powder inject...
Embodiment 3
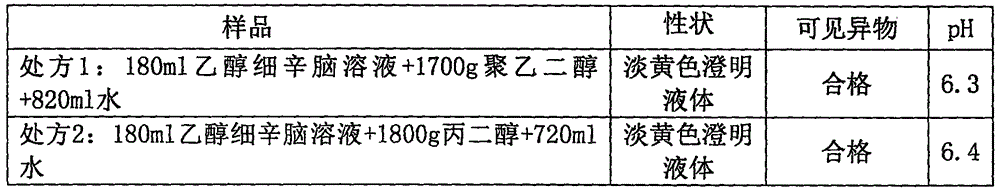
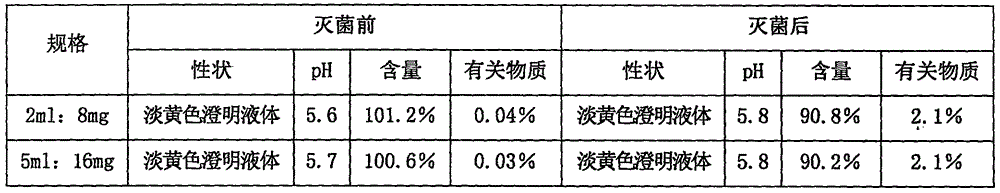
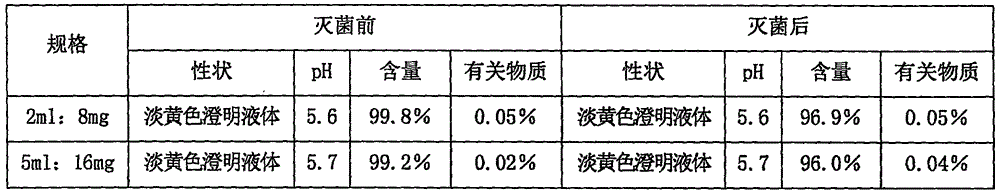
[0055] Embodiment 3 asarone injection
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com