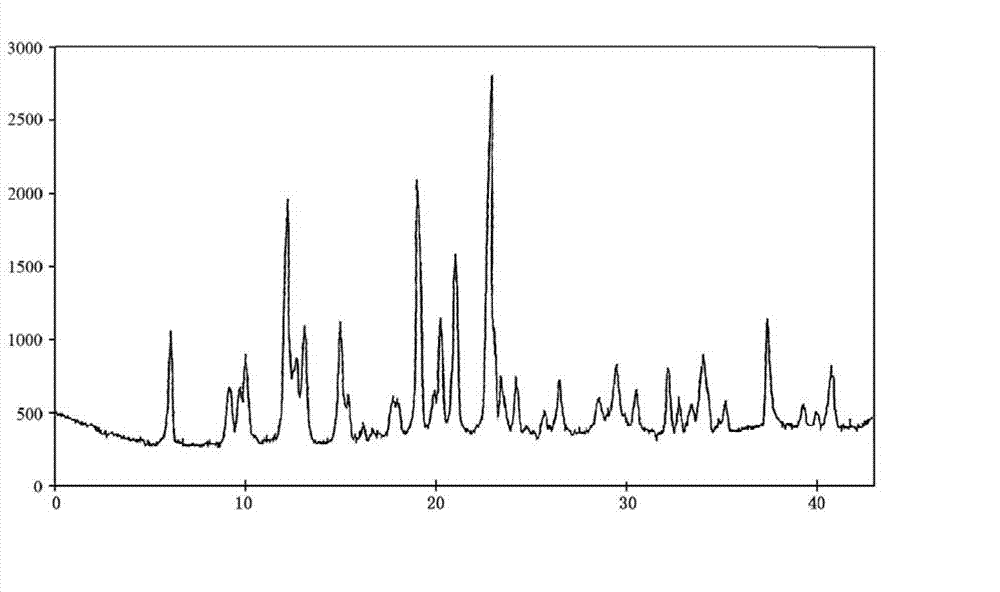
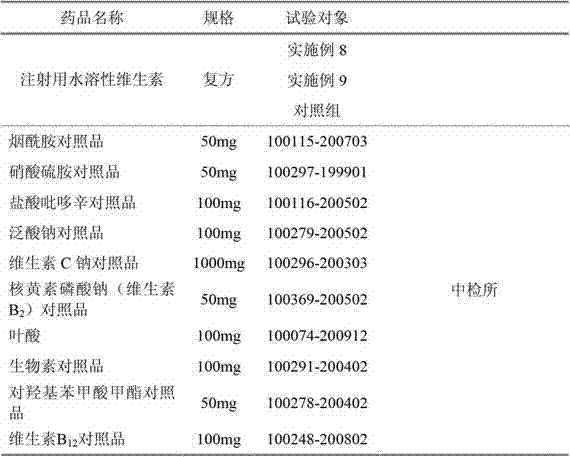
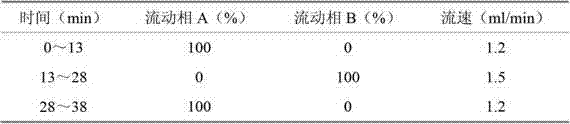
Water-soluble vitamin freeze-dried preparation for injection and preparation method thereof
A technology for water-soluble vitamins and freeze-dried preparations, which can be used in freeze-dried delivery, medical preparations containing active ingredients, pharmaceutical formulas, etc. The effect of improving purity and yield, ensuring drug safety, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 Preparation of sodium pantothenate compound
[0043] (1) Take the commercially available crude product of sodium pantothenate, add water equivalent to 40 times the weight of the crude product of sodium pantothenate, stir and dissolve at 50°C, and concentrate under reduced pressure to 1 / 5 of the original volume; among them, concentrate under reduced pressure under vacuum at 60 Below ℃, the vacuum degree is 0.07 MPa;
[0044] (2) Add activated carbon 0.08 times the weight of crude sodium pantothenate to the solution for decolorization, and filter;
[0045] (3) Stir and add ethanol:ether mixed solution whose volume is 1 / 4 of the solution to the filtrate; the volume of ethanol and ether in the ethanol:ether mixed solution is 1:8. The speed of / min cools the filtrate to 12°C; wherein, the stirring speed is 8rmp;
[0046] (4) Stop stirring, cool the solution down to 2°C at a uniform speed within 30 minutes, and place it under an ultrasonic field to grow crystal...
Embodiment 2
[0048] Embodiment 2 Preparation of sodium pantothenate compound
[0049] (1) Take the commercially available crude sodium pantothenate, add water equivalent to 30 times the weight of the crude sodium pantothenate, stir and dissolve at 45°C, and concentrate under reduced pressure to 1 / 4 of the original volume; wherein, concentrate under reduced pressure under vacuum at 60 Below ℃, the vacuum degree is 0.06 MPa;
[0050] (2) Add activated carbon 0.05 times the weight of crude sodium pantothenate to the solution for decolorization, and filter;
[0051] (3) Stir and add ethanol:ether mixed solution whose volume is 1 / 5 of the solution to the filtrate; the volume of ethanol and ether in the ethanol:ether mixed solution is 1:5. The speed of / min cools the filtrate to 10°C; wherein, the stirring speed is 6rmp;
[0052] (4) Stop stirring, cool the solution down to 0°C within 20 minutes, and place it in an ultrasonic field to grow crystals for 6 hours, the ultrasonic frequency is 0.1K...
Embodiment 3
[0053] Embodiment 3 Preparation of sodium pantothenate compound
[0054] (1) Take the commercially available crude sodium pantothenate, add water equivalent to 45 times the weight of the crude sodium pantothenate, stir and dissolve at 55°C, and concentrate under reduced pressure to 1 / 6 of the original volume; wherein, concentrate under reduced pressure under vacuum at 60 Below ℃, the vacuum degree is 0.08 MPa;
[0055] (2) Add activated carbon 0.1 times the weight of the crude product of sodium pantothenate to the solution for decolorization, and filter;
[0056] (3) Stir and add ethanol:ether mixed solution whose volume is 1 / 3 of the solution to the filtrate; the volume of ethanol and ether in the ethanol:ether mixed solution is 1:12 while feeding at 2.5°C / The speed of min will cool the filtrate to 15°C; wherein, the stirring speed is 12rmp;
[0057] (4) Stop stirring, cool the solution down to 5°C within 40 minutes, and place it under an ultrasonic field to grow crystals ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com