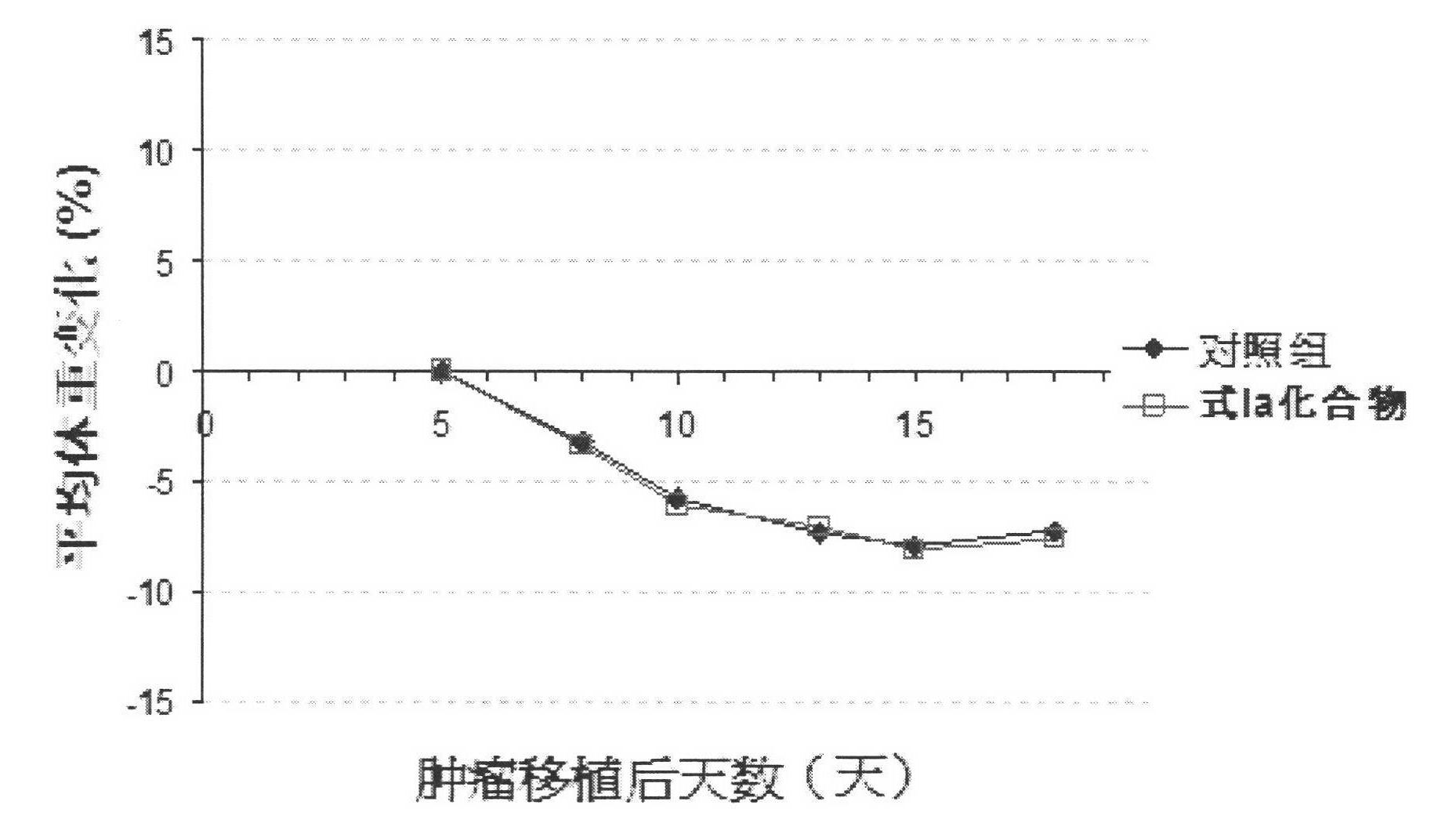
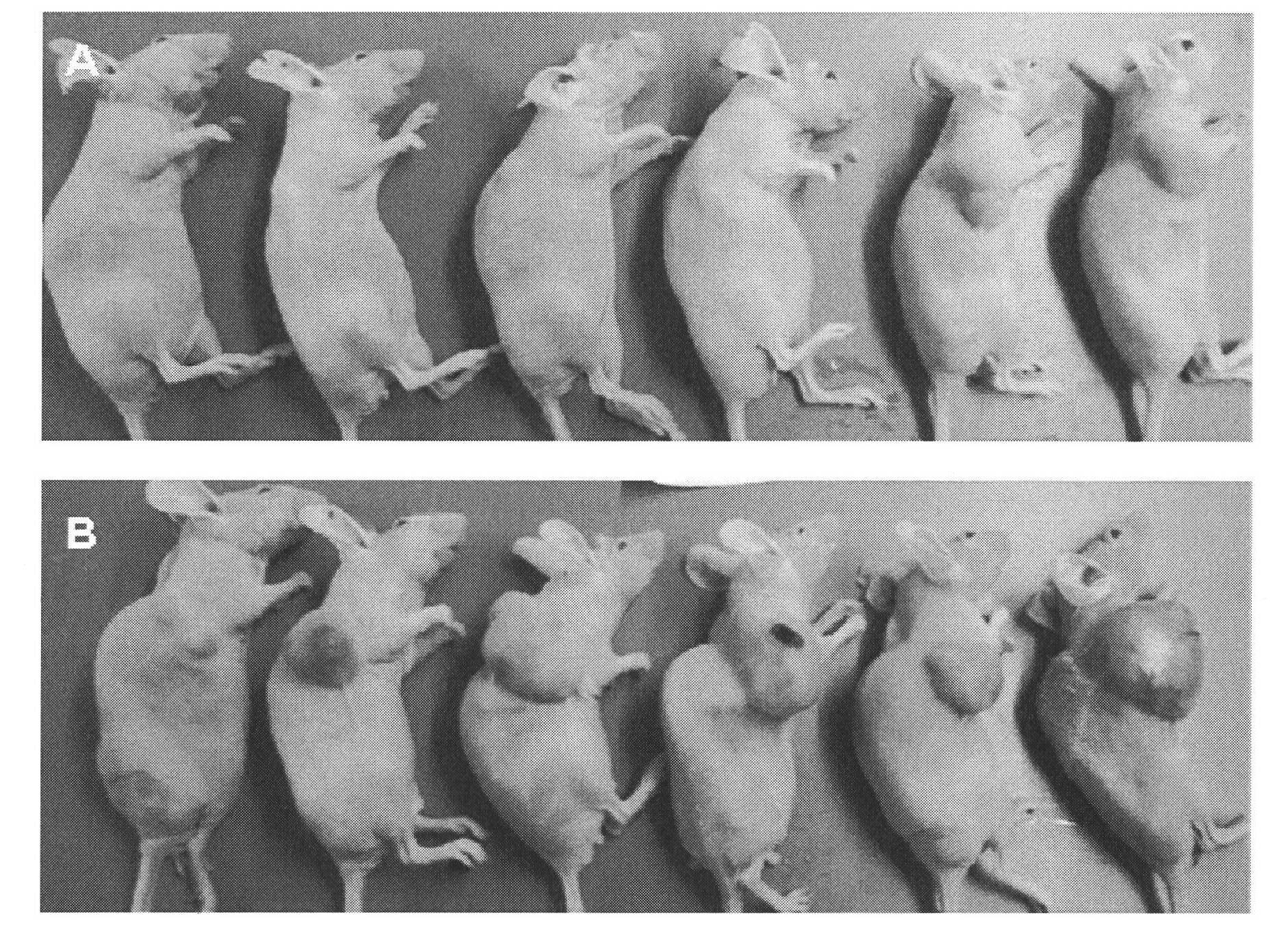
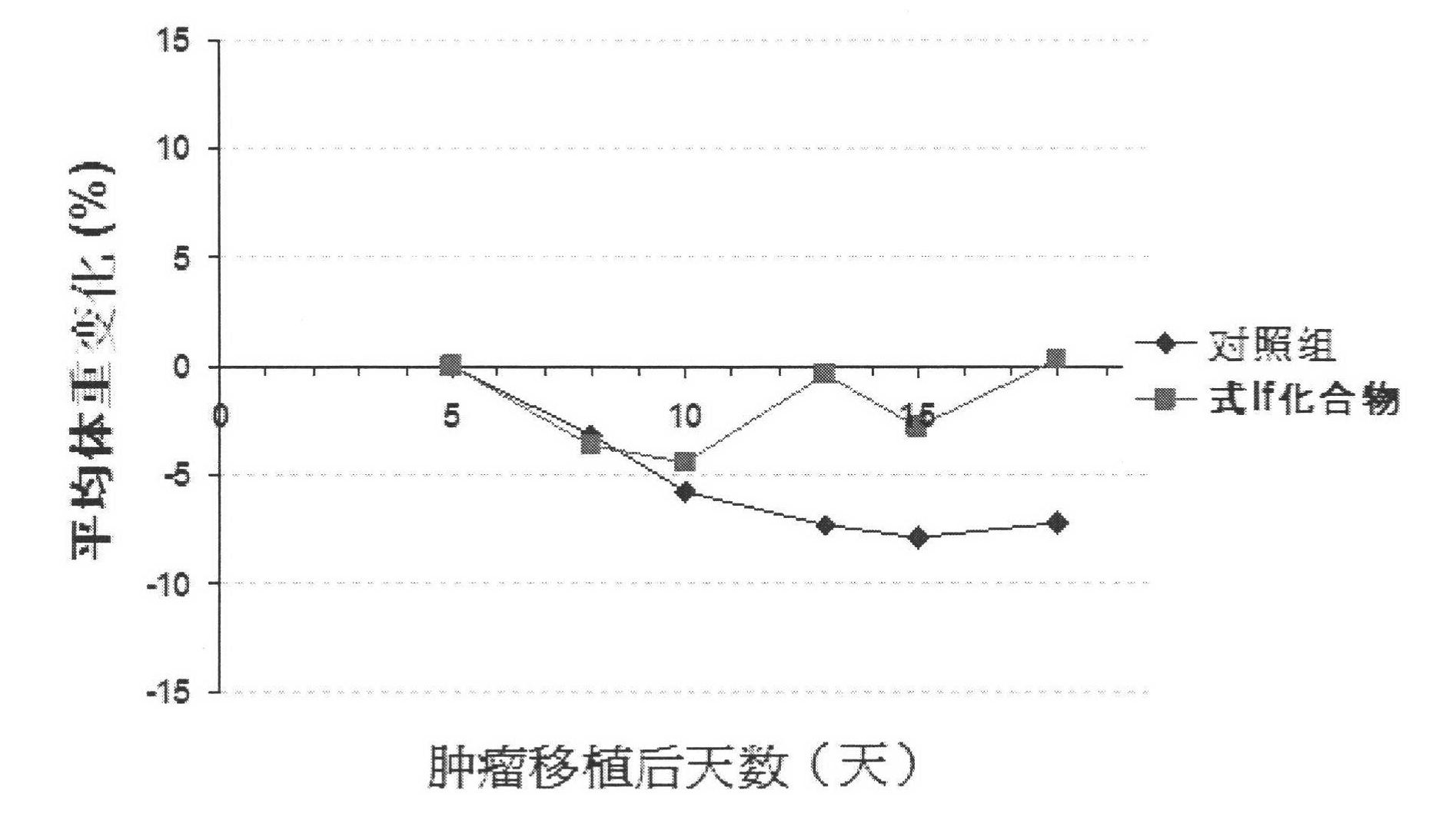
Thiazoleamide compound and use thereof for the preparation of anti-malignant tumor medicines
A technology of thiazole amides and compounds, which is applied in the field of preparation of anti-malignant tumor drugs, can solve the problems of increasing side effects, low water solubility, affecting bioavailability, etc., and achieves the effects of easy preparation, high bioavailability, and improved effective bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Formula Ia compound, its chemical name is: N-(2-chloro-6-methylphenyl)-2-[[6-[4-tert-butoxycarbonyl-2,2,3,3,5,5, 6,6-Octadeutero-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide.
[0039]
[0040] The compound of formula Ia can be obtained by the following synthetic route:
[0041]
[0042] (1), preparation of 1-tert-butoxycarbonyl-deuterated piperazine: at room temperature, slowly add an aqueous solution of sodium hydroxide (0.78g, 19.5mmol) 1ml to deuterated piperazine hydrochloride while stirring (3.25g, 19.4mmol), reacted for about 5 minutes, then added dropwise 250ml of methanol, stirred for 30 minutes, reacted in an ice-salt bath at -20°C for 30 minutes, and slowly added di-tert-butyl dicarbonate dropwise About 50ml of methanol solution (4.25g, 19.5mmol), put it at room temperature and react for 1 hour, concentrate, add water and stir for 15 minutes, then filter, the filtrate is extracted with dichloromethane, and the organic phase is drie...
Embodiment 2
[0049] Compound of formula Ib: N-(2-chloro-6-methylphenyl)-2-[[6-[4-ethoxycarbonyl-2,2,3,3,5,5,6,6-octadeuterium -1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide.
[0050]
[0051] The compound can be obtained by making N-(2-chloro-6-methyl-phenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazolecarboxamide with 1- Ethoxycarbonyl-deuterated piperazine is obtained through a substitution reaction, and the specific preparation process can be found in Example 1.
Embodiment 3
[0053] Compound of formula Ic: N-(2-chloro-6-methylphenyl)-2-[[6-[4-tert-butylcarbonyl-2,2,3,3,5,5,6,6-octadeuterium -1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, the structural formula is as follows:
[0054]
[0055] The compound can be obtained by making N-(2-chloro-6-methyl-phenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5thiazolecarboxamide with 1-tert Butylcarbonyl-deuterated piperazine is obtained through a substitution reaction, and the specific preparation process can be found in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com