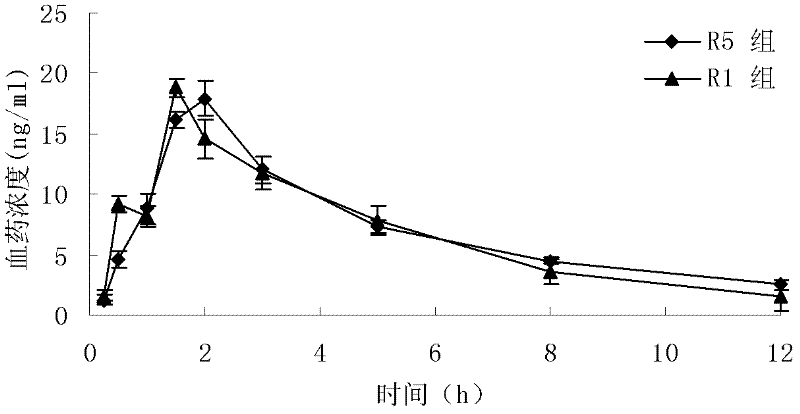
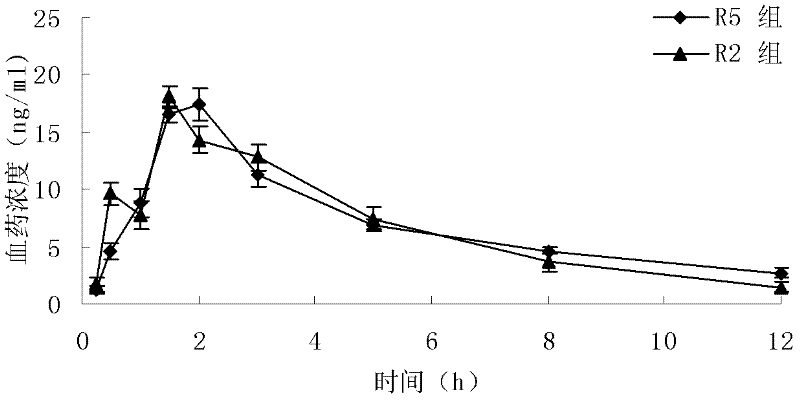
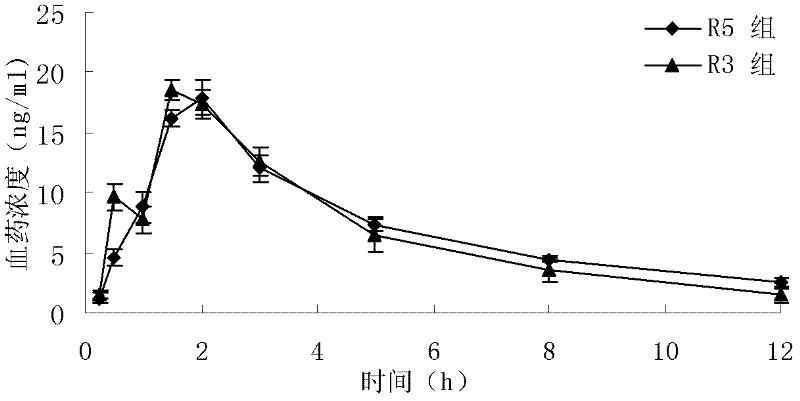
Loratadine lyophilized tablets and preparation method thereof
A technology of loratadine and dry tablets, which is applied in the field of loratadine freeze-dried tablets and its preparation, can solve the problems of obvious side effects and low bioavailability, and achieve the effect of no gritty feeling and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] The preparation formula of the present invention is made up of following components:
[0106]
[0107]The specific preparation method is as follows: add loratadine, glycine, and pullulan to the fully dissolved xanthan gum solution, and mix to form a uniform solution; after the solution is degassed, it is accurately injected into the mold; After pre-freezing at -40°C to -170°C for 1 to 60 minutes, transfer to a freeze dryer, and freeze-dry at a pressure of 0.01 mbar to 10 mbar and at a temperature of -30°C to 30°C for 1 to 10 hours to obtain this product Invented loratadine freeze-dried tablets; after the pre-freezing step in the above method, the step of ice crystal incubation can also be added before being transferred to the lyophilizer, that is, the mold filled with the solution after the pre-freezing is put into -5 ℃ ~ -60 In the low temperature environment of ℃, the time is 5~15h.
Embodiment 2
[0109] The preparation formula of the present invention is made up of following components:
[0110]
[0111] The specific preparation method is as follows: add the loratadine, mannitol, and pullulan in the above-mentioned amounts into the fully dissolved xanthan gum solution, and mix to form a homogeneous solution; the rest of the preparation method is the same as in Example 1.
Embodiment 3
[0113] The preparation formula of the present invention is made up of following components:
[0114]
[0115] The specific preparation method is as follows: add the above-mentioned amount of loratadine, glycine, mannitol, pullulan, sodium alginate, and peppermint essence to the fully dissolved konjac gum solution, and mix to form a uniform solution; All the other preparation methods are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com