Propofol ester derivative containing amino carboxylic acid amide structure, its preparation method and its purpose
A technology of aminocarboxylic acid amide and propofol, which is applied to the preparation of carboxylic acid amide, the preparation of organic compounds, and medical preparations containing active ingredients, etc. It can solve the problems of poor water solubility, achieve stable aqueous solution, and have cheap and easy-to-obtain raw materials , reducing the effect of the first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
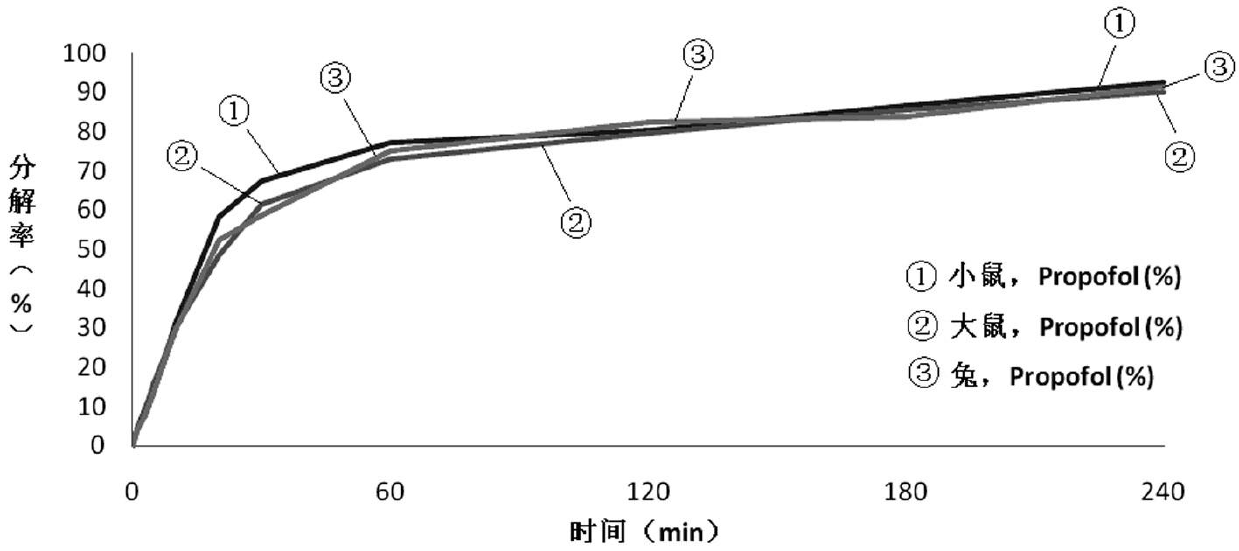
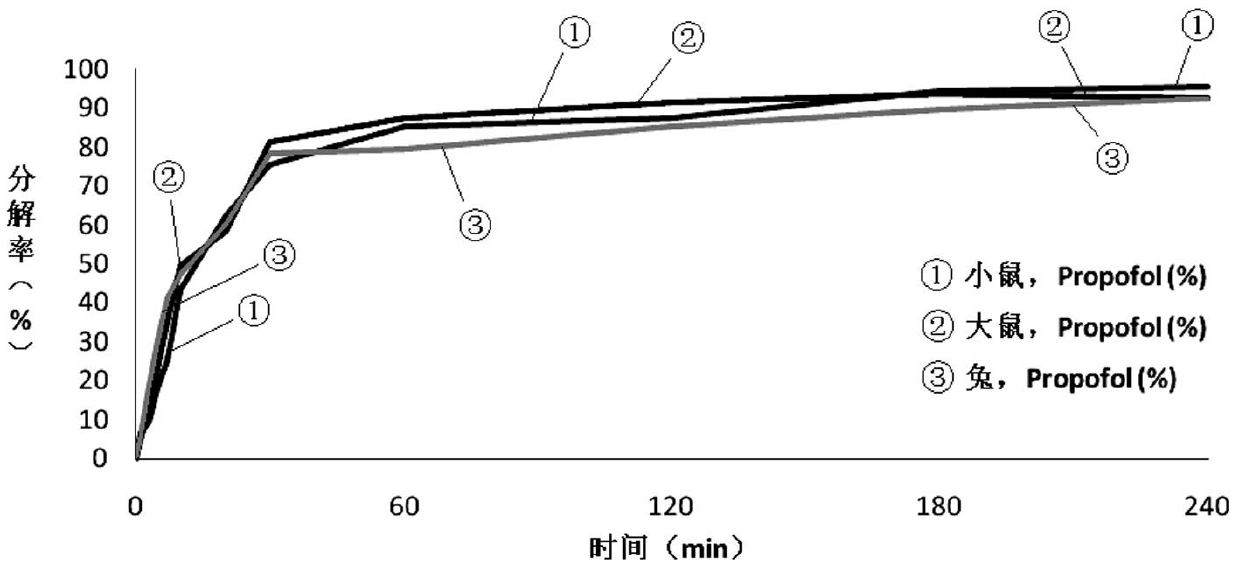
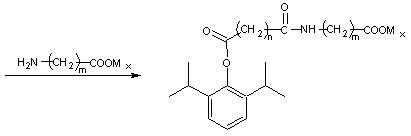
Image
Examples
Embodiment 1
[0039] Dissolve 20 g of propofol (formula II) in 50 ml of triethylamine, add 14 g of succinic anhydride and 0.02 g of dimethylaminopyridine, stir at room temperature for 16 h, and distill the reaction solution under reduced pressure to remove excess triethylamine , the residue was washed into 100 ml of water, and the pH value was adjusted to 1 with 6 N hydrochloric acid, a large amount of white precipitate was precipitated, filtered and dried under reduced pressure to obtain the crude product of propofol succinic acid monoester intermediate (Ⅲ), washed with cyclohexane / Recrystallization from ethyl acetate gave 23.5 g of needle-like crystals, the reaction yield was 75.4%, and mp: 103-104°C.
Embodiment 2
[0041] Dissolve 20 g of propofol in 100 ml of dichloromethane, add 13.3 g of succinic acid and 0.02 g of dimethylaminopyridine, then add 23.2 g of DCC and stir at room temperature for 6 h, then filter off the white solid in the reaction solution , the filtrate was washed once with 150 ml of 6N hydrochloric acid, the organic layer was separated, and the solvent was evaporated under reduced pressure to obtain a light yellow solid of propofol succinate monoester intermediate (Ⅲ), which was recrystallized from cyclohexane / ethyl acetate 26.6 g of white needle-like crystals were obtained, the yield was 85%, and mp: 102-103°C.
Embodiment 3
[0043] Weigh 1 g of the propofol succinic acid monoester intermediate (Ⅲ) of Example 1 into a 25 ml single-necked bottle, add 10 ml of dry dichloromethane, and quickly add 2 g of freshly distilled oxalyl chloride, and pass through a drying tube React at room temperature for 2 h. After the HCl is completely released, evaporate the solvent and low-volatile substances to dryness on a rotary evaporator to obtain the crude propofol succinic acid monoester chloride, which can be used directly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com