99mTc complex, preparation method, intermediate and application thereof
A 99mtc, 99mtco4- technology, applied in the direction of radioactive carriers, can solve the problem of low labeling rate, achieve the effect of simple steps, good imaging prospect, and enhanced imaging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
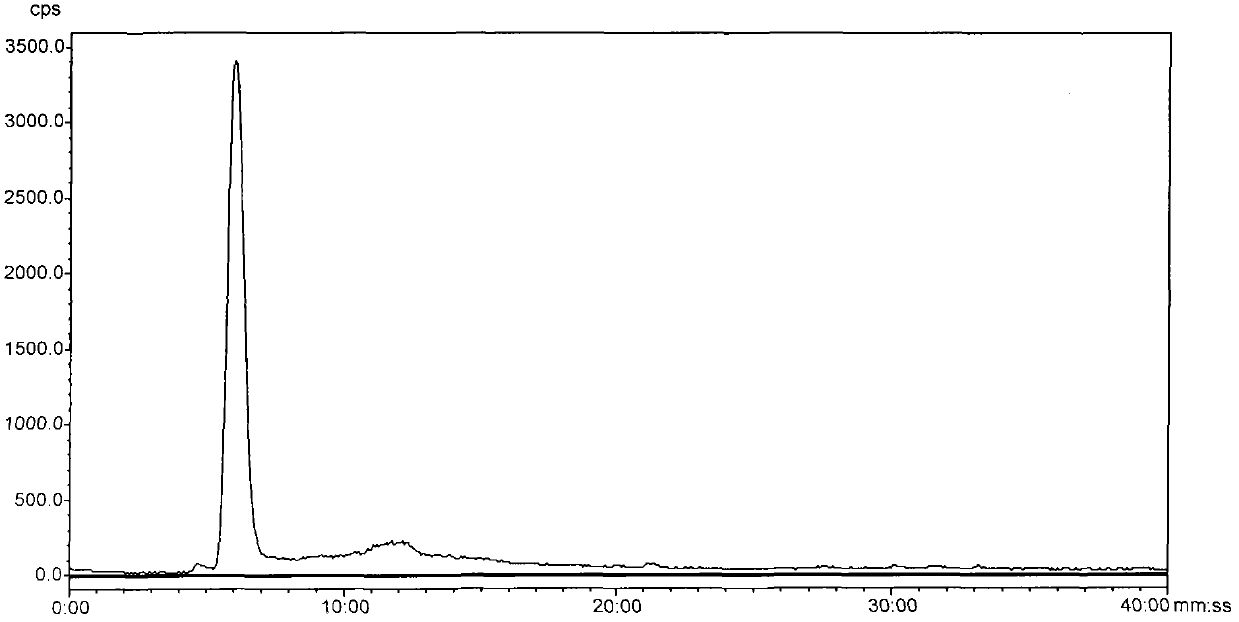
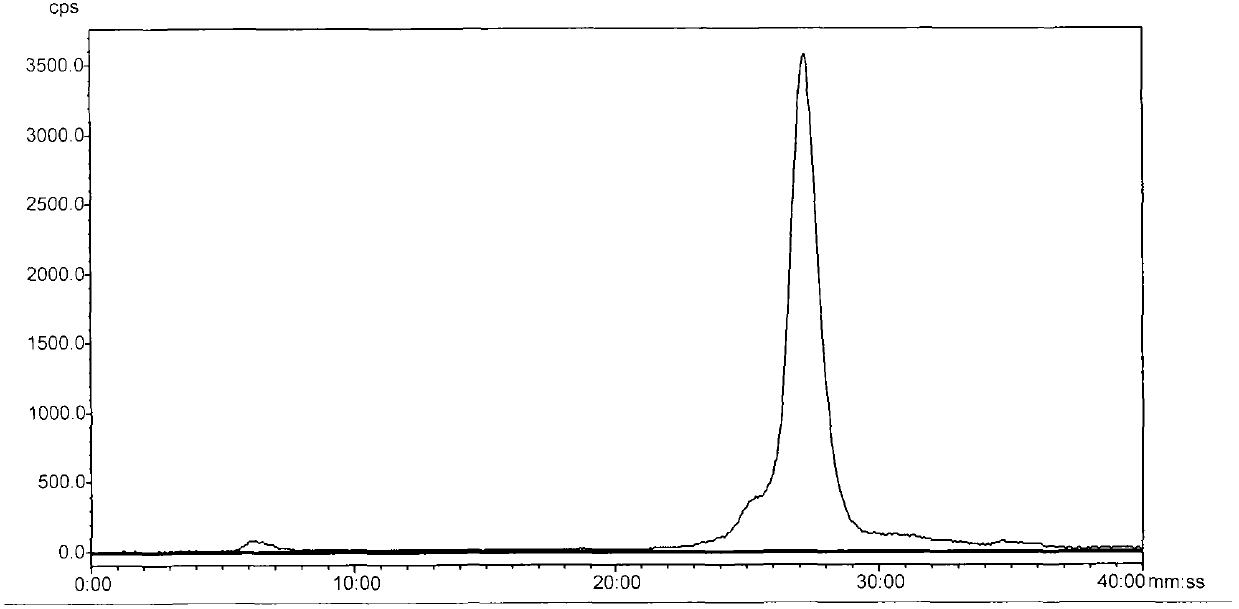
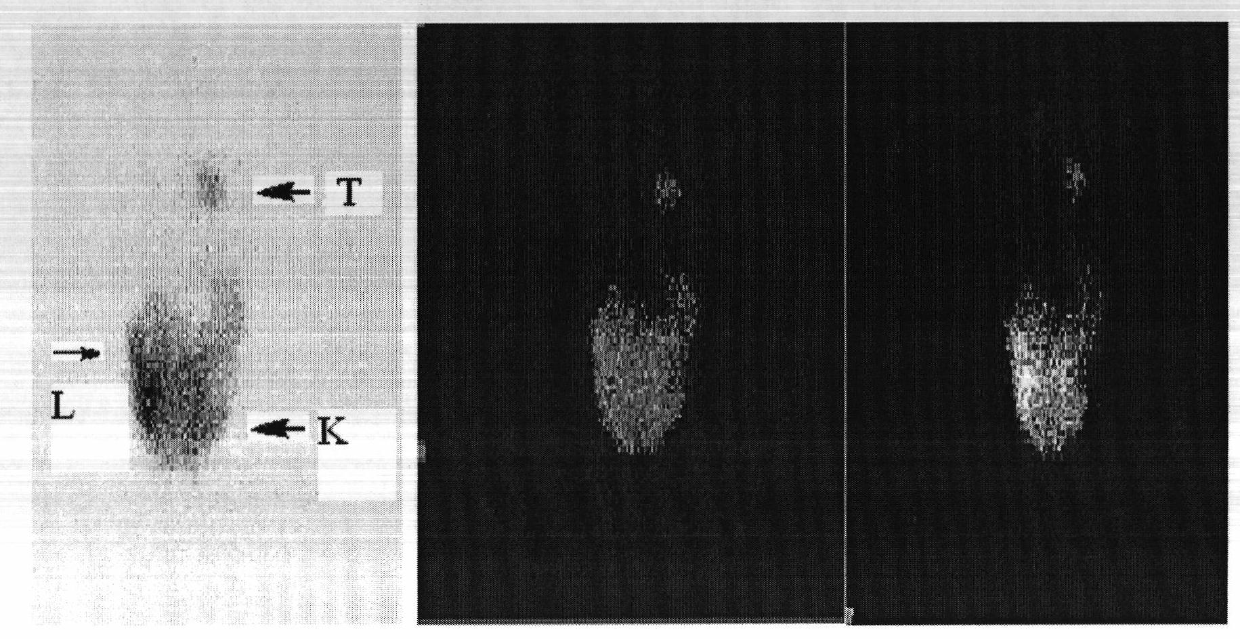
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of Compound V (n=77) (Partial Acetylation of Fifth Generation Polyamido-amine Dendrimers)
[0052]
[0053] According to the literature (Istva'n J.Majoros, Acetylation of Poly(amidoamine) Dendrimers, Macromolecules 2003,36,5526-5529) acetylation dendrimers, the specific steps are as follows: 100mg fifth generation polyamide-amine dendrimers (compound Ⅵ, G5PAMAM, molecular weight 28824g / mol, stored in methanol, wt: 5%) (about 3.47×10 -6 mol), 24.9μl acetic anhydride (Ac 2 O) (about 2.43×10 -4 mol) and 43.4μl triethylamine (Et 3 N) (about 3.04×10 -4 mol) were mixed, stirred and reacted for 24 h at room temperature (25° C.) under nitrogen protection, and a colorless transparent liquid was obtained. Spin to dry the solvent, dissolve the product with deionized water, filter with a water-phase needle filter (specification 25mm×0.20μm), and dialyze the filtrate in PBS solution (PBS formula: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO 4 1.44g, KH 2 PO 4 0.27...
Embodiment 2
[0056] Example 2: Synthesis of Compound III (m=7, n=77, p=10) (Synthesis of PAMAM-Ac-pegFa)
[0057]
[0058] 0.090g compound VII (about 3.27×10 -5 mol) was dissolved in 3ml DMSO, at 15°C, a solution of 3.27×10 -6 mol PAMAM-Ac in 3ml DMSO solution, reaction 3d. A yellow transparent solution was obtained. The solvent was spin-dried, and the product was dissolved in deionized water, filtered through a needle filter (25 mm×0.20 μm) of the aqueous phase, and the filtrate was dialyzed in PBS solution for one day, and the PBS solution was replaced three times. The above product was continued to be dialyzed in deionized water for two days, and the deionized water was changed three times a day. After the dialysis, the obtained product was freeze-dried, and the yield was about 93.7%.
[0059] Its identification data are as follows:
[0060] 1 H-NMR (D 2 O, TMS, 500MHz), δ1.89(230H, s), 2.28(504H, s), 8.64(7H), 7.62(14H), 6.68(14H)
Embodiment 3
[0061] Example 3: Synthesis of Compound I (m=7, n=77, p=10) (Synthesis of PAMAM-Ac-pegFa-DTPA)
[0062]
[0063] containing approximately 2.94 x 10 -6 mol PAMAM-Ac-pegFa (compound III) in the aqueous solution (5mL), add 0.0572g compound IV (about 2.94×10 -5 mol), adjust the pH value to about 9 with NaOH solution (1M). At 40°C, reacted for 24h to obtain a yellow transparent solution. The solvent was spin-dried, and the product was dissolved in deionized water, filtered through a needle filter (25 mm×0.20 μm) of the aqueous phase, and the filtrate was dialyzed in PBS solution for one day, and the PBS solution was replaced three times. The above product was continued to be dialyzed in deionized water for two days, and the deionized water was changed three times a day. After the dialysis, the obtained product was freeze-dried, and the yield was about 94.4%.
[0064] The NMR data are as follows:
[0065] 1 H-NMR (D 2O, TMS, 500MHz), δ1.88 (230H, s), 2.33 (504H, s), 6.78 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com