Carbazochrome sodium sulfonate sodium chloride injection and preparation method thereof
A technology of sodium carboxysulfonate sodium chloride and sodium carboxysulfonate sodium chloride, applied in the field of medicine, can solve problems such as hidden dangers in product quality and safety, incomplete low-temperature sterilization, unstable product storage for a long time, etc., and achieve product quality and safety. The effect of stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
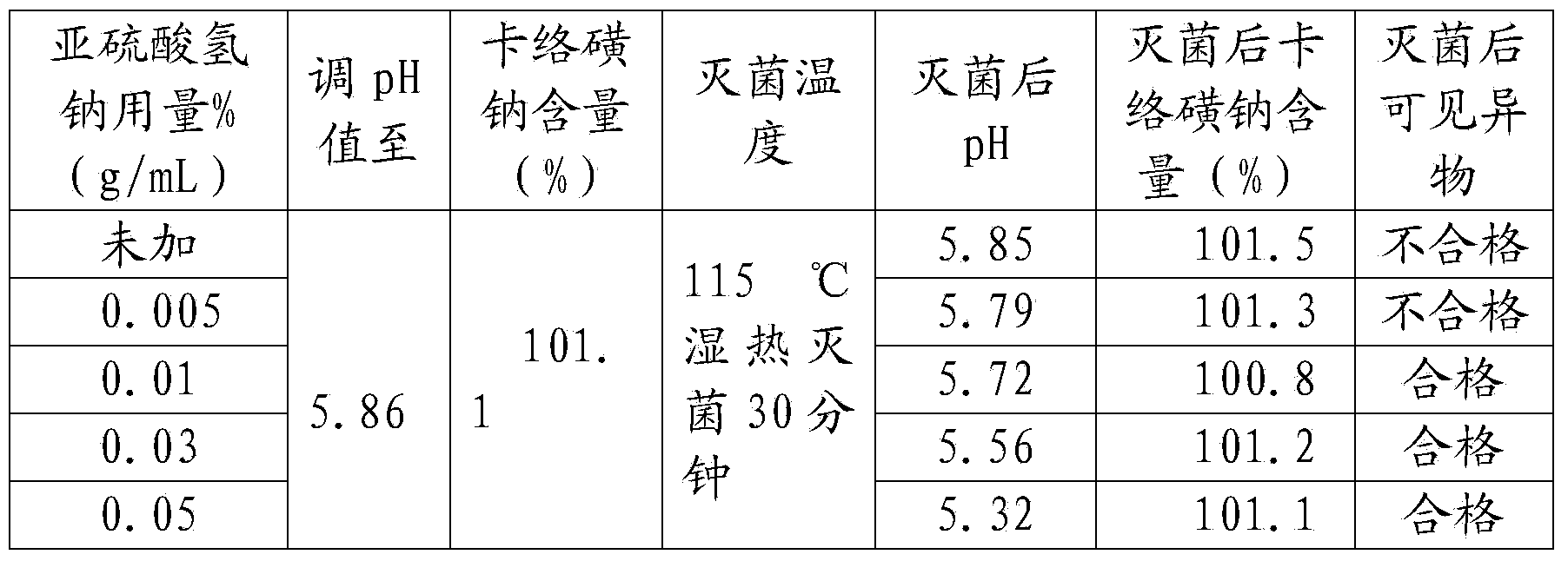
[0039] A kind of sodium carbosulfonate sodium chloride injection, comprising in every liter of injection: 0.8 g of sodium carbosulfonate, 9 g of sodium chloride, 0.05 g of disodium edetate, 0.5 g of disodium hydrogen phosphate, and sodium bisulfite 0.1g, activated carbon 0.1g;
[0040] The specific steps of the preparation method include:
[0041] (1) Take sodium carbosulfonate, sodium chloride, disodium edetate, disodium hydrogen phosphate, sodium bisulfite, activated carbon respectively according to the formula ratio, and set aside;
[0042] (2) Add water for injection with a volume of 20% in the concentrated preparation tank, stir, add sodium chloride, edetate disodium, and disodium hydrogen phosphate, stir and dissolve, and filter to the dilute preparation tank;
[0043] (3) Add water for injection to 95% of the volume of the prepared volume in the dilute preparation tank, and at the same time pass in cooling water to reduce the temperature to 45°C-50°C, pass in filtered ...
Embodiment 2
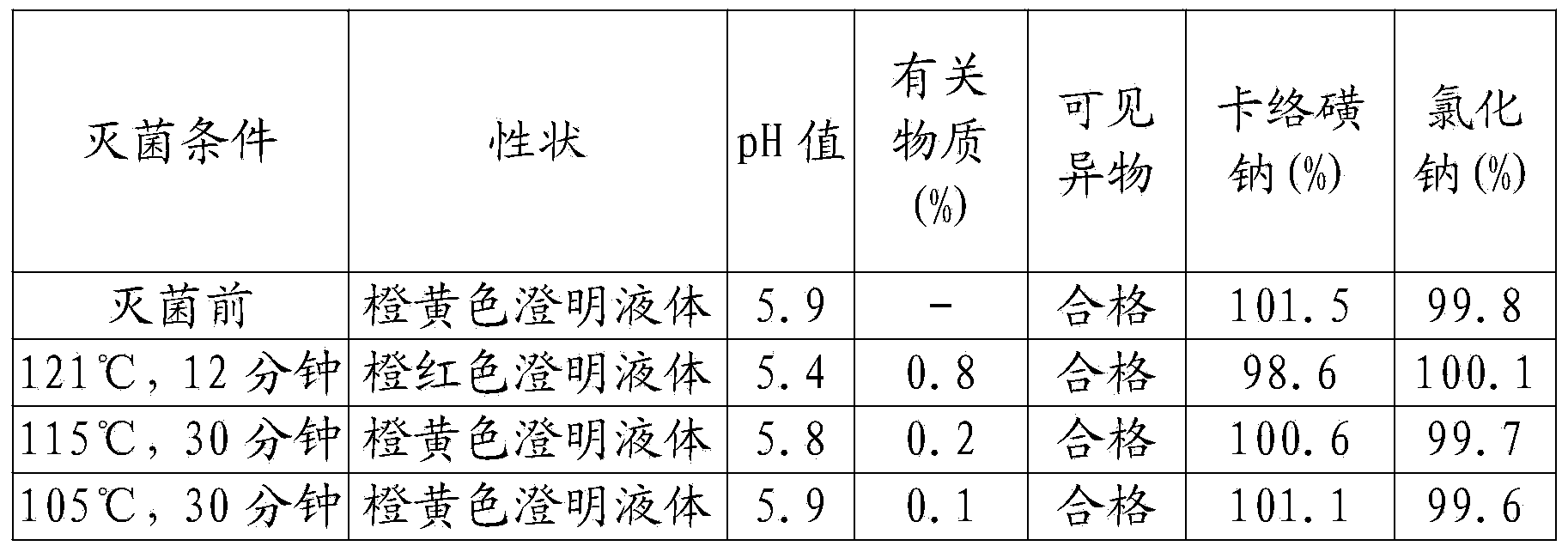
[0046] A kind of sodium carbosulfonate and sodium chloride injection, comprising in every liter of injection: 0.5 g of sodium carbosulfonate, 8 g of sodium chloride, 0.02 g of disodium edetate, 0.8 g of sodium citrate, 0.3 g of sodium sulfite, activated carbon 0.5g;
[0047] The specific steps of the preparation method include:
[0048] (1) Take sodium carbosulfonate, sodium chloride, disodium edetate, sodium citrate, sodium sulfite, and activated carbon respectively according to the formula ratio, and set aside;
[0049] (2) Add water for injection with a volume of 30% in the concentrated preparation tank, stir, add sodium chloride, edetate disodium, and sodium citrate, stir and dissolve, and filter to the thin preparation tank;
[0050] (3) Add water for injection to 95% of the volume of the prepared volume in the dilute preparation tank, and at the same time pass into cooling water to reduce the temperature to 50°C-55°C, pass through filtered nitrogen, and adjust with 0.1m...
Embodiment 3
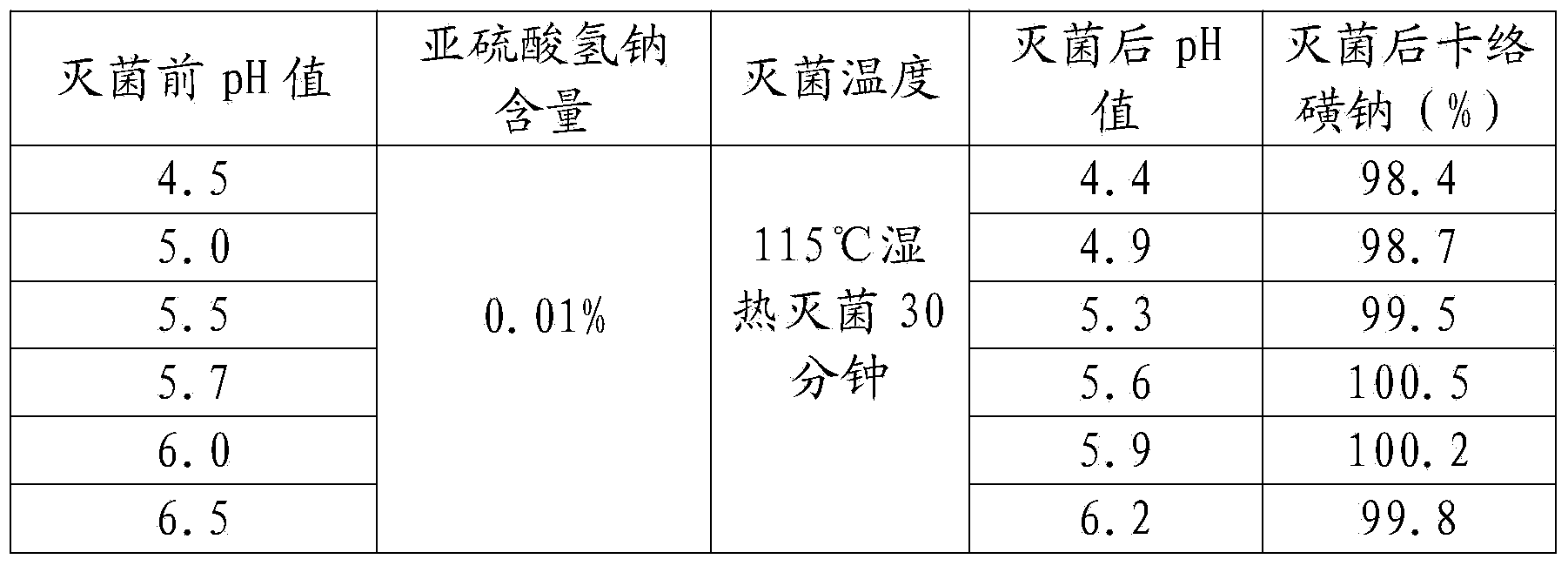
[0053] A kind of sodium carbosulfonate and sodium chloride injection, comprising in every liter of injection: 0.9 g of sodium carbosulfonate, 5 g of sodium chloride, 0.1 g of disodium edetate, 0.1 g of sodium acetate, 0.5 g of sodium metabisulfite, activated carbon 1g;
[0054] The specific steps of the preparation method include:
[0055] (1) Take sodium carbosulfonate, sodium chloride, edetate disodium, sodium acetate, sodium metabisulfite, activated carbon respectively by weighing according to the formula ratio, and set aside;
[0056] (2) Add water for injection of 40% of the prepared volume into the thick preparation tank, stir, drop into sodium chloride, edetate disodium, sodium acetate, after stirring and dissolving, filter to the thin preparation tank;
[0057] (3) Add water for injection to 95% of the volume of the prepared volume in the dilute preparation tank, and at the same time, pass in cooling water to reduce the temperature to 55°C-60°C, pass in filtered nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com