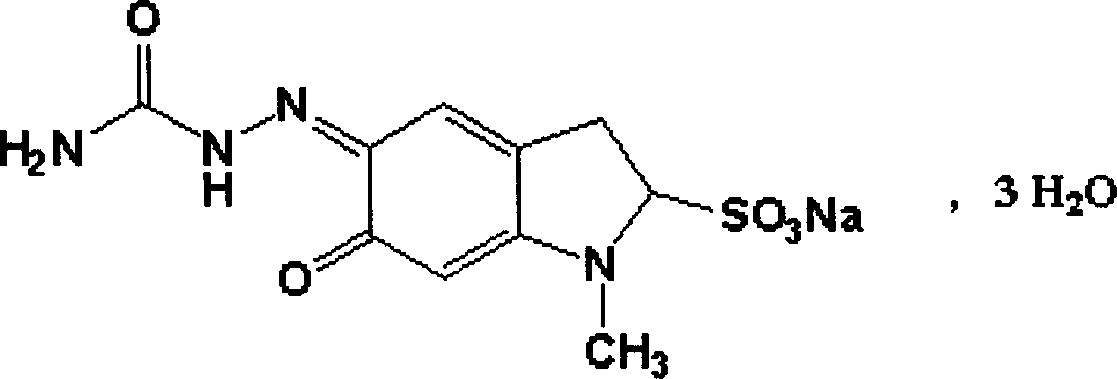
Carbazochrome sodium sulfonate slow-released tablet and its preparing method
A technology of sodium carboxysulfonate and sustained-release tablets, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of poor compliance, inconvenience in using injections, and low blood drug concentration And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Sodium Carbosulfonate Hydrophilic Gel Matrix Tablet
[0046] prescription:
[0047] Sodium carbosulfonate 30g
[0048] HPMC K4M 50g
[0049] Lactose 80g
[0050] 95% ethanol appropriate amount
[0051] Magnesium Stearate 1.5g
[0053] Preparation Process:
[0054] Take carbosulfonium sodium raw material, pulverize and pass through a 120-mesh sieve, mix evenly with HPMC K4M and lactose, moisten with 95% ethanol and granulate, dry at 50°C and granulate, add magnesium stearate and talc, Mix evenly and tablet.
[0055] For the release test, the release rate of the sample was measured according to the first method of Appendix XC of the Chinese Pharmacopoeia in 2005, Part Two of the Chinese Pharmacopoeia Edition. First use 900ml of dilute hydrochloric acid (9→1000) as solvent, rotate at 100 rpm, take samples at 1 and 2 hours, then change to 900ml of buffer salt with pH 6.8 as solvent, operate in the same way, take samples at 4 and 8...
Embodiment 2
[0061] Example 2 Sodium carbene sulfonate waxy matrix tablet
[0062] prescription:
[0063] Sodium carbosulfonate 30g
[0064] Octadecanol 30g
[0065] Lactose 90g
[0066] Magnesium Stearate 1.5g
[0068] Preparation Process:
[0069] The crude drug of sodium carbosulfonate is crushed and passed through a 120-mesh sieve, mixed evenly with lactose, molten stearyl alcohol is added therein, mixed evenly and then granulated, magnesium stearate and talcum powder are added, mixed evenly and pressed into tablets.
[0070] The release test is the same as in Example 1, and the results are as follows:
[0071] Time (hours) cumulative release %
[0072] 1 26.8%
[0073] 2 39.3%
[0074] 4 65.1%
[0075] 8 94.3%
Embodiment 3
[0076] Example 3 Sodium carbosulfonate non-erodible matrix tablet
[0077] prescription:
[0078] Sodium carbosulfonate 30g
[0079] Ethylcellulose 45g
[0080] Lactose 75g
[0081] 95% ethanol appropriate amount
[0082] Magnesium Stearate 1.5g
[0083] Talc powder 1.5g
[0084] Preparation Process:
[0085] The crude drug of sodium carbosulfonate is crushed and passed through a 120-mesh sieve, mixed evenly with lactose, ethyl cellulose, magnesium stearate and talcum powder, and the whole powder is directly compressed into tablets.
[0086] The release test is the same as in Example 1, and the results are as follows:
[0087] Time (hours) cumulative release %
[0088] 1 23.4%
[0089] 2 36.9%
[0090] 4 60.3%
[0091] 8 87.6%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com