Freeze-dried injection containing carbazochrome sodium sulfonate and method of preparing the same
A technology of freeze-dried powder injection and sodium carbosulfonate, which is applied in the field of pharmaceutical preparations, can solve problems such as redissolution, and achieve the effects of rapid dissolution, stable product quality, and good solution clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
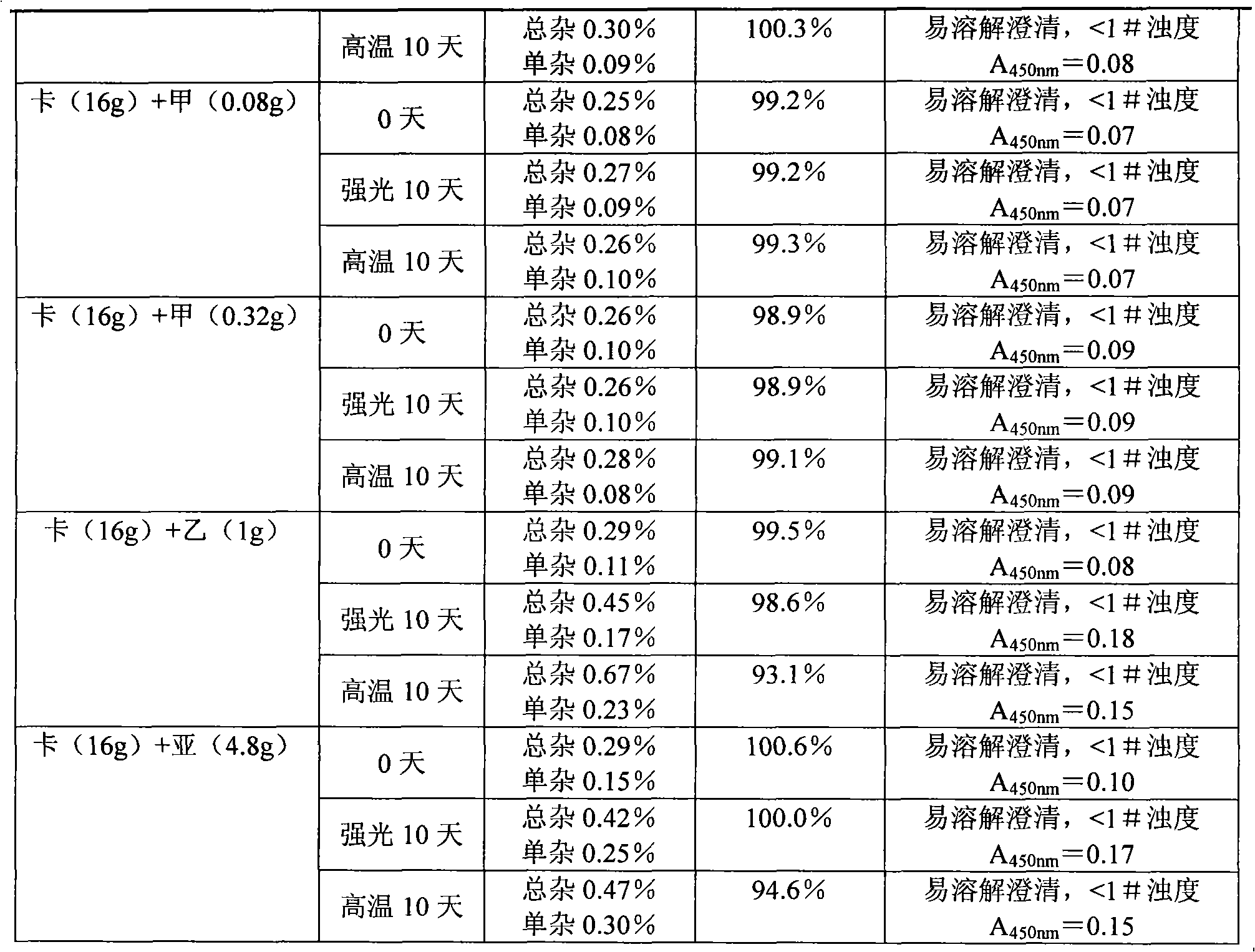
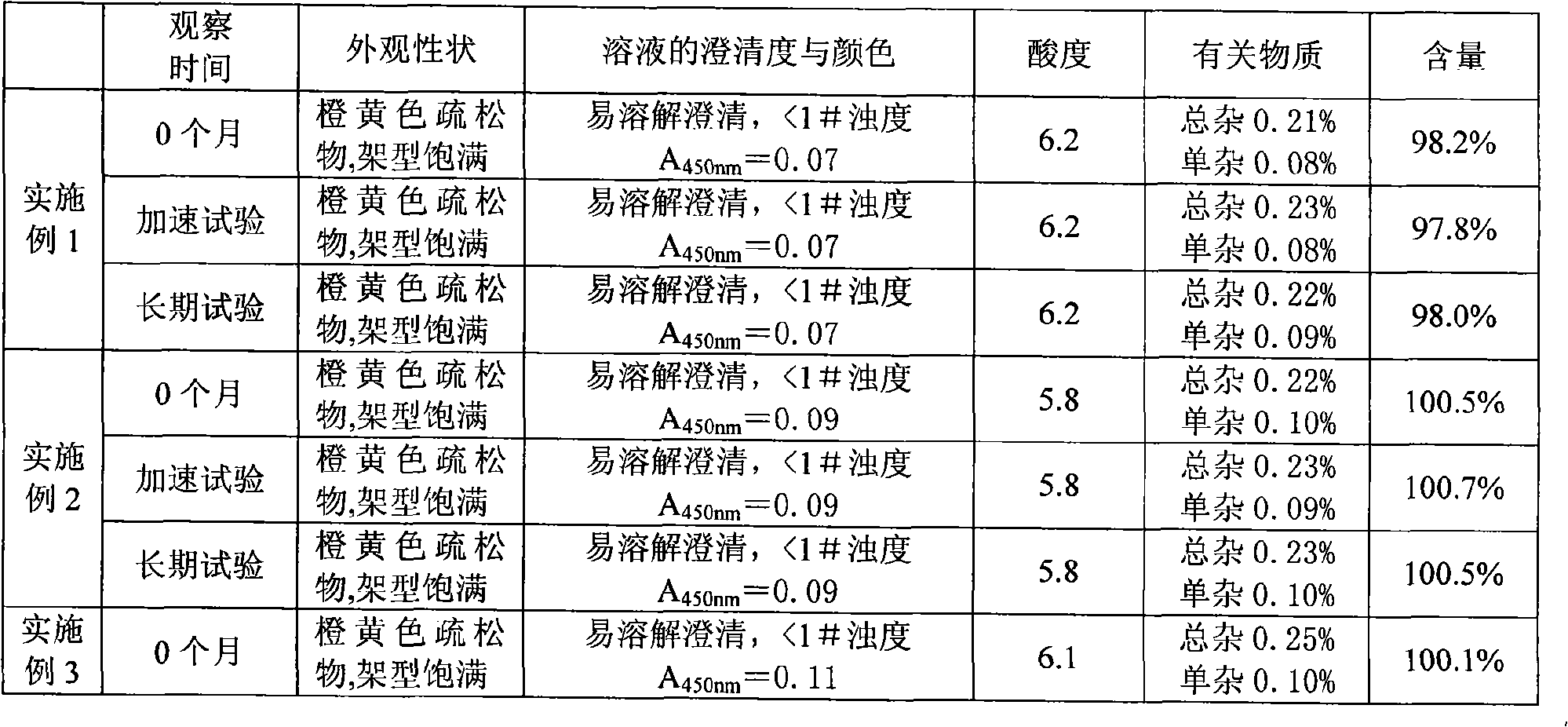
Embodiment 1
[0032] Component Weight (according to 1000 count)
[0033] Sodium carbosulfonate 20g
[0034] Mannitol 6g
[0035] Thiourea 0.1g
[0036] Preparation process: Mix the above three components, add 500ml of water for injection to dissolve, and dilute the water for injection to 1000ml so that the concentration of the sodium carbosulfonate solution is 2%. Sterilize and filter with a 0.22 μm microporous membrane, fill in a container with a filling volume of 1 ml / bottle, freeze-dry, press stopper, crimp the cap, and pack.
Embodiment 2
[0038] Component Weight (according to 1000 count)
[0039] Sodium carbosulfonate 20g
[0040] Mannitol 14g
[0041] Dextran 4g
[0042] Sodium formaldehyde sulfoxylate 0.2g
[0043] The preparation method is the same as in Example 1.
Embodiment 3
[0045] Component Weight (according to 1000 count)
[0046] Sodium carbosulfate 40g
[0047] Mannitol 28g
[0048] Xylose 8g
[0049] Sodium formaldehyde sulfoxylate 0.4g
[0050] Thiourea 0.2g
[0051] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com