Carbazochrome sodium sulfonate pharmaceutical composition with good stability and high safety as well as preparation method and application thereof
The technology of sodium carboxylate and composition is applied in the field of sodium carboxylate pharmaceutical composition and preparation thereof, which can solve the problems of intramuscular injection and the like, and achieve the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation of embodiment 1 formula III compound
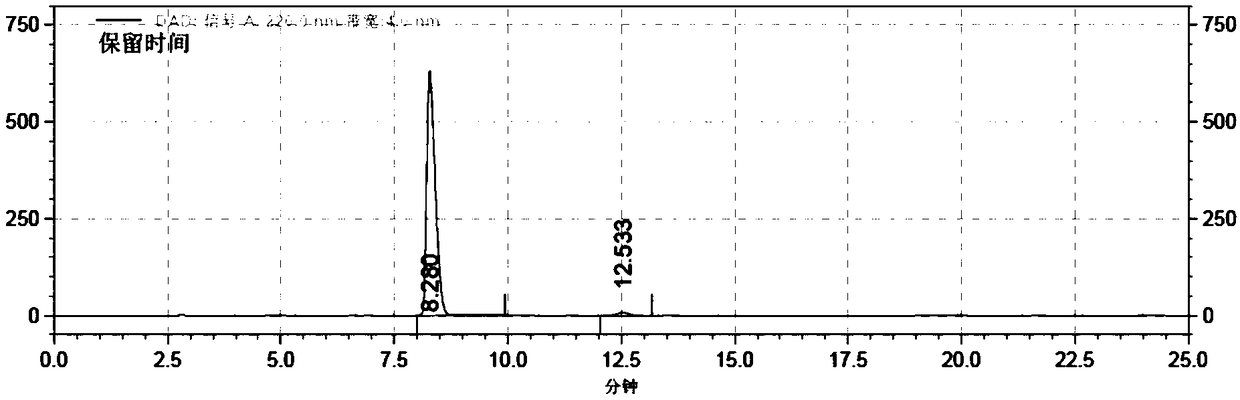
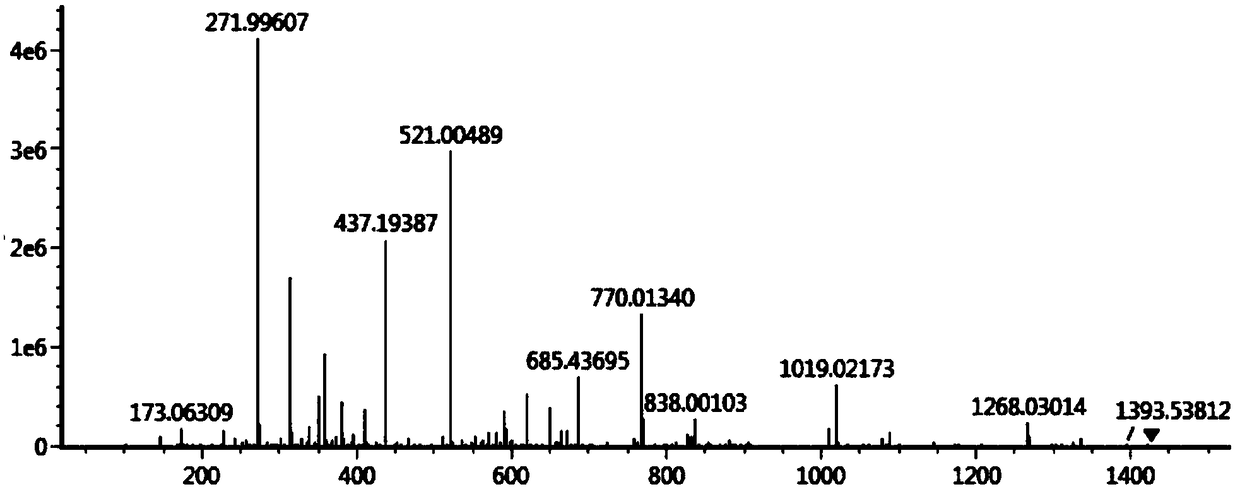
[0060] Take 10g of sodium carbosulfonate, add 500ml of 50% ethanol aqueous solution, heat and stir to dissolve, add 10ml of triethylamine, reflux in water bath for 60 minutes, and use preparative high-efficiency liquid phase separation for the reflux liquid, and the mobile phase is acetonitrile-ammonium dihydrogen phosphate buffer Liquid, receive product fraction, promptly obtain 0.1g formula III compound. HPLC peak area normalization method, the measured content is not less than 98%. MS (high resolution mass spectrum) is 271.9961 ([M+Na] + ).
[0061] Table 1 HPLC determination result table
[0062] Retention time (minutes)
Peak area(%)
8.280
1109907760
98.61
12.533
15674886
1.39
total
1125582646
100.00
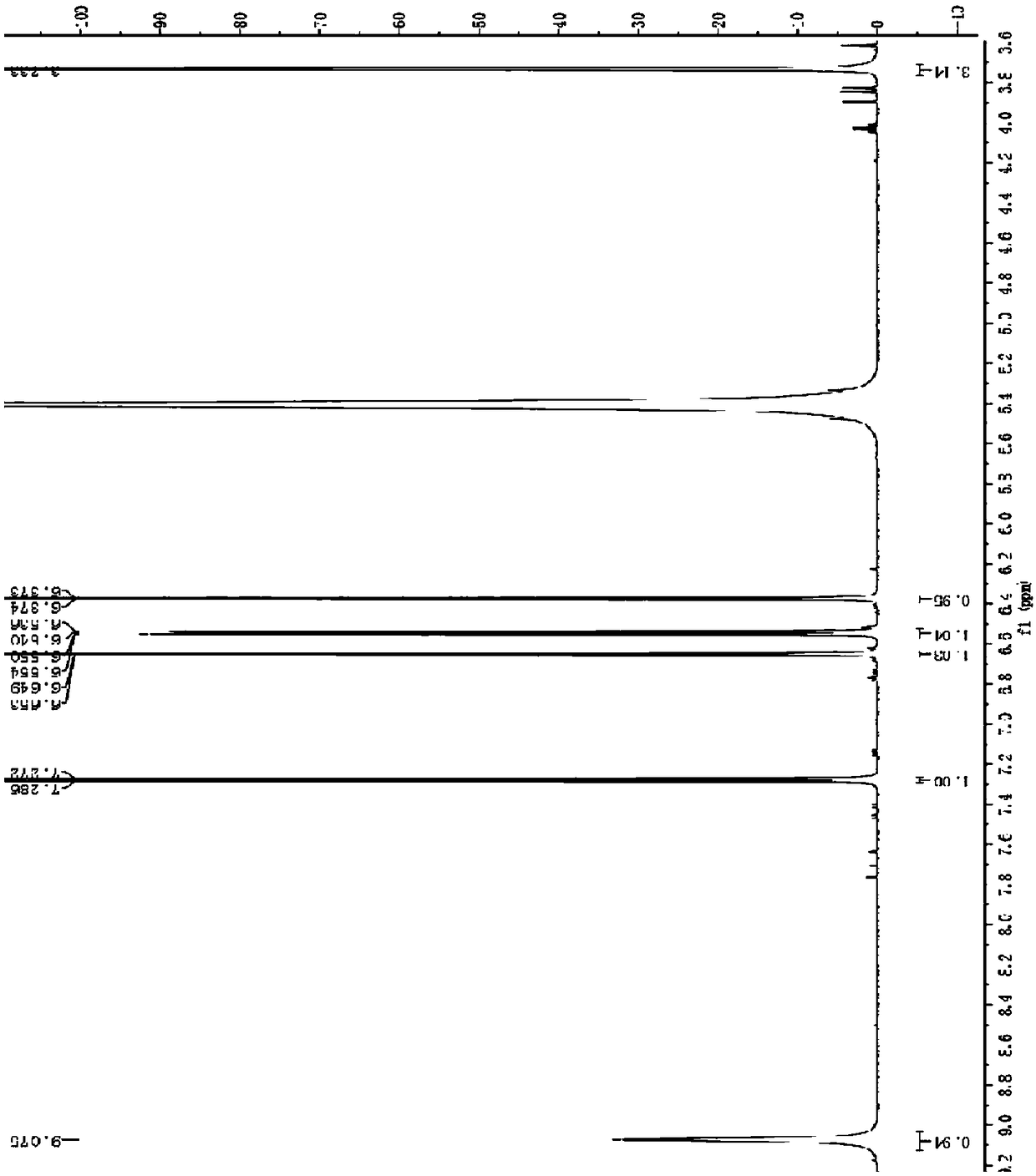
[0063] Table 2 1 H NMR and 1 H- 1 H COZY measurement analysis table
[0064]
[0065] The preparation of embodiment 2 f...
Embodiment 3
[0069] Example 3 Toxicity study of compound of formula III
[0070]Using L929 cell line (ATCC CCL1 [NCTC clone 929 (mouse fibroblast)]), sodium carbosulfate (provided by Jiangsu Hanstone Pharmaceutical Co., Ltd., refined to a purity of 99.9%, does not contain the compound of formula III ) and the compound of formula III (the present invention, with a purity of 99.9%) were incubated with L929 cells for 24h, 48h, and 72h (final concentrations were respectively 10, 5, 2.5, 1.25, 0.625, 0.3125, 0.15625, and 0.078125mg / ml), using The cell viability of each test product at different time points was measured by MTT Assay, and the IC50 value of the half maximal inhibitory concentration at each time point was calculated by Bliss method. Results: the IC50 value of sodium carbosulfonate was 3.87 mg / ml, and the IC50 value of the compound of formula III was 0.02 mg / ml. Result shows that the IC50 value of carbosulfonate sodium is about 194 times of formula III compound, and the cytotoxic...
Embodiment 4
[0071] Example 4 Toxicity Study of Sodium Carbosulfonate and Its Composition
[0072] Adopt L929 cell line, test drug carbosulfonate (provided by Jiangsu Hanstone Pharmaceutical Co., Ltd., refined into 99.9% purity, does not contain formula III compound) and formula III compound (the present invention, purity 99.9%) Composition groups with weight ratios of 1:0.1%, 1:0.25%, 1:0.5%, 1:0.75%, 1:1%, 1:1.5%, and 1:2% (hereinafter referred to as "compositions") Before administration, the test drug was dissolved in water for injection, and diluted to the required concentration according to the dosage requirements (wherein the final concentration of sodium carbosulfonate was 1mg / ml), and the colorimetric method of tetramethylazozolium salt (MTT) was adopted. Assay) measured the cell viability of each test product at different time points.
[0073] Table 4 Cytotoxic reaction grading judgment table
[0074] level
Relative survival rate (%)
0
≥100
1
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com