Formula of oral mucosa absorbing dosage form of medicines of polypeptides and proteins, and preparation method and application thereof
A technology of oral mucosa and absorbents, which is applied in the formulation, preparation and application of oral mucosa absorption dosage forms of polypeptide and protein drugs, and can solve the problems of difficult curative effect of polypeptide and protein drugs, short action time of chewing gum, and patch The problem of limited drug loading can achieve the effect of improving bioavailability, avoiding drug loss, and simplifying the composition of the prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
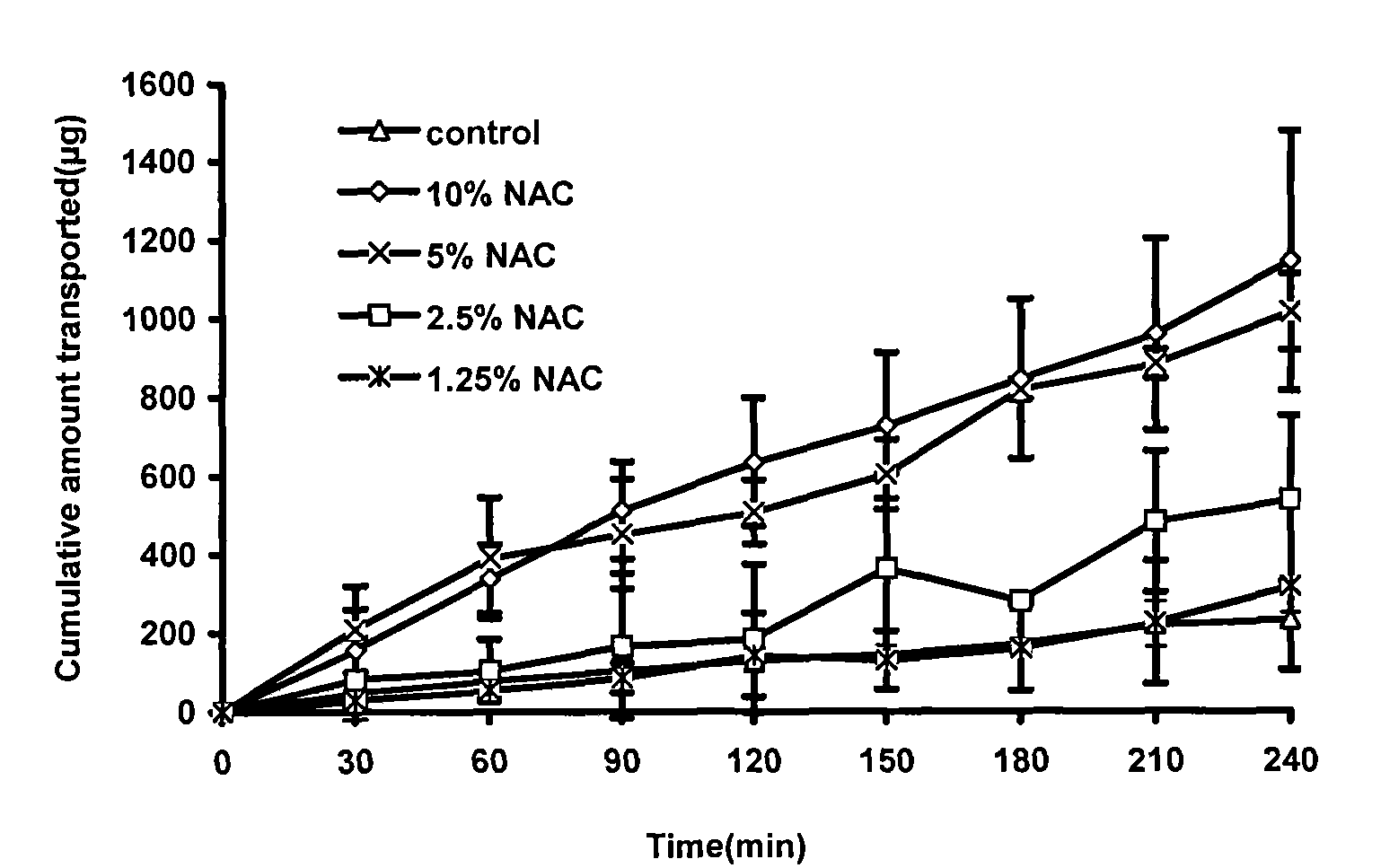
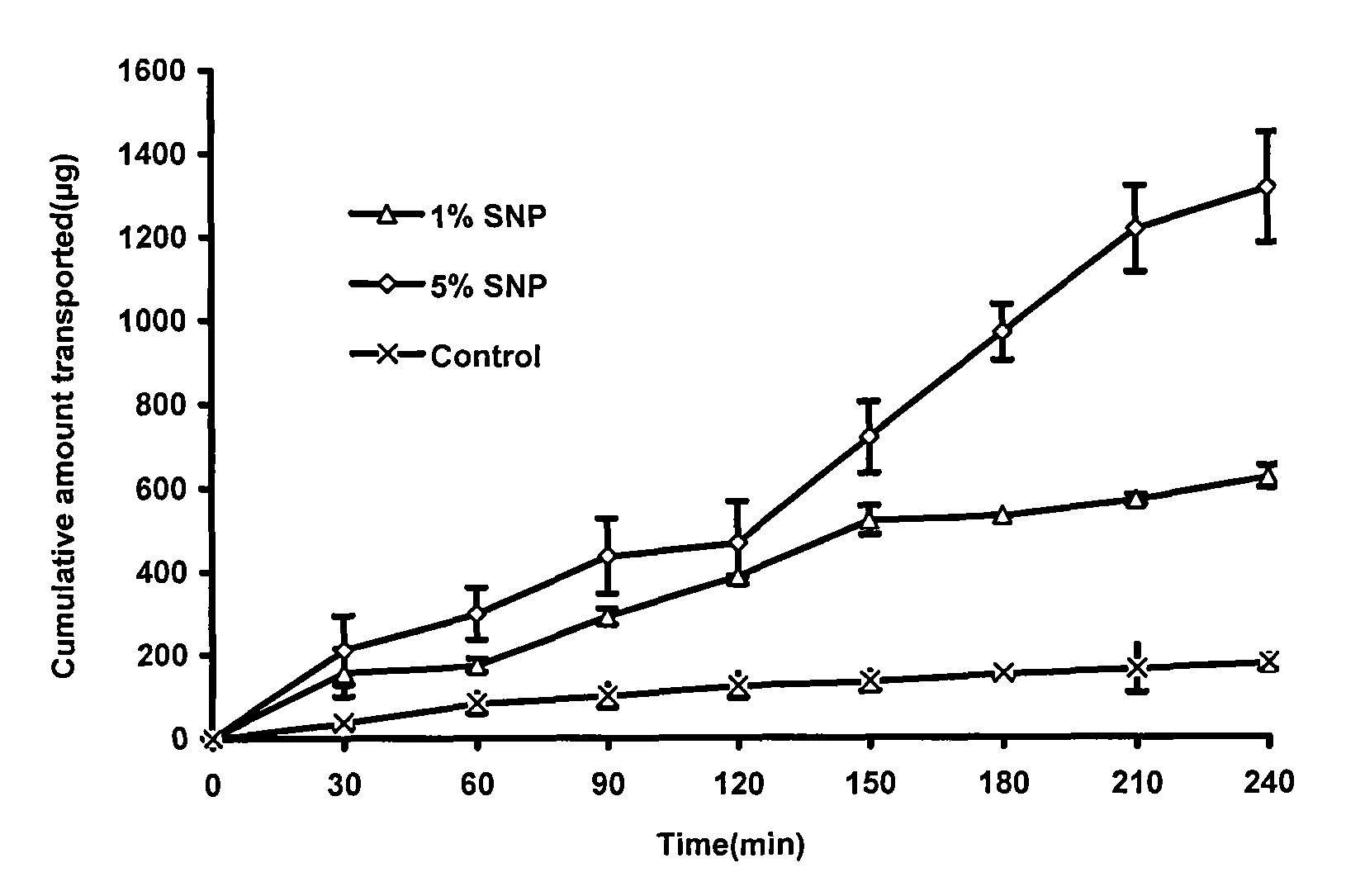
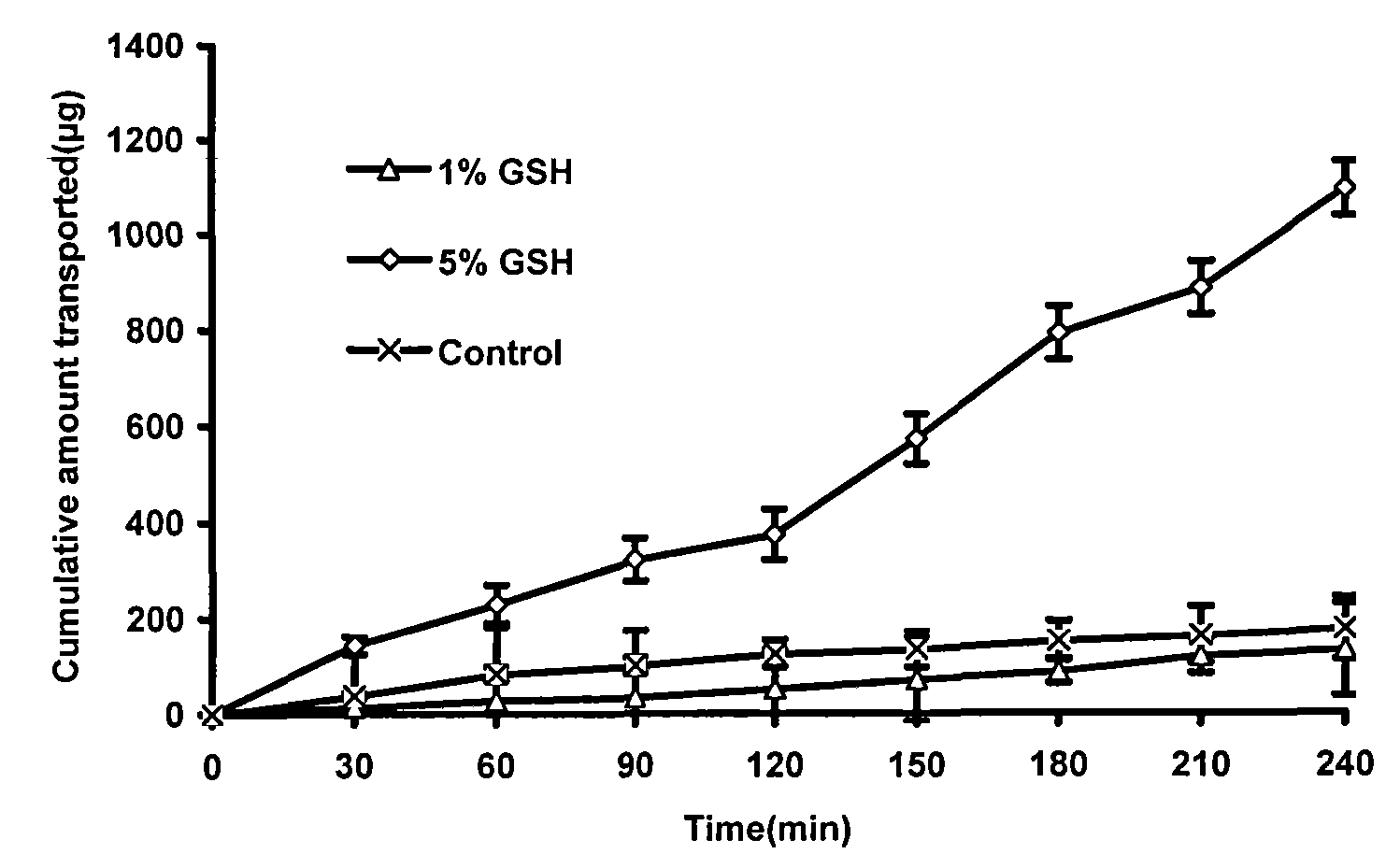
[0066] Embodiment 1 screens penetration-enhancing absorbent
[0067] The TR146 oral mucosal epithelial cell osmotic transport model was used to study the absorption promotion. Aspirate the DMEM culture solution in the upper and lower chambers of the insert culture dish, and wash the TR146 cells with HBSS buffer. Then add HBSS solution into the upper and lower chambers respectively, after equilibrating for 1 hour, suck out the HBSS solution from the upper chamber, and add 2mL of the test solution (containing different concentrations of osmosis-absorbing agents). Take a sample of 100 μL from the lower chamber at the set time, and immediately add an equal amount of HBSS solution. Using chromatographic method, sample injection analysis, record the obtained peak area (A), and calculate the cumulative penetration of insulin at each time point. Calculate the following indicators according to the obtained data: ①Insulin apparent permeability coefficient: P app =dQ / dt×1 / (A×C 0 ); ②...
Embodiment 2
[0075] The cytotoxic effect of embodiment 2 permeation-enhancing absorption agent
[0076] Using TR146 cells in the exponential growth phase, prepare 2×10 4 cells / mL single-cell suspension, 100 μL per well was inoculated in a 96-well plate. After 48 hours, 80 μL of culture medium was supplemented, and then 20 μL of insulin-free test solution was administered. The preparation method of the test solution was the same as that in 2.2. After incubation for 48 hours, MTT detection was carried out at a detection wavelength of 490 nm. Taking the enzyme activity of untreated cells as 100%, calculate the enzyme activity of the cells treated with the test solution. Calculation formula: relative enzyme activity (%)=(OD 1 -OD 2 ) / (OD 3 -OD 4 ), OD 1 Refers to the OD value of the cells treated with the test solution, OD 2 Refers to the OD value of the test solution without cells, OD 3 Refers to the OD value of untreated cells, OD 4 Refers to the OD value of the blank well when the...
Embodiment 3
[0078] Preparation of Example 3 Insulin Oral Adhesive Tablet
[0079] Prescription Composition of Insulin Oral Adhesive Tablets (mg / tablet)
[0080]
[0081] According to the prescription design in the table, mix the solutions of various auxiliary materials evenly and pour them into the mold. The mold was pre-frozen in a -20°C refrigerator for 24 hours, and then freeze-dried for 24 hours with a freeze dryer to remove the solvent component in the solution, and the adhesive layer of the insulin oral adhesive sheet was obtained. Then, smear a waterproof layer on it, the waterproof layer is 10% ethyl cellulose solution (prepared with dehydrated alcohol), placed at room temperature until dehydrated alcohol evaporates completely, promptly obtains the insulin oral cavity adhesive double-layer tablet, puts in dry Store in a refrigerator at 4°C. See the preparation process Figure 7 .
[0082] Figure 7 It is a schematic diagram of the preparation method of insulin oral adhesiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com