Porous composite medicine-loaded composition for improving oral absorption for probucol, and preparation method of porous composite medicine-loaded composition
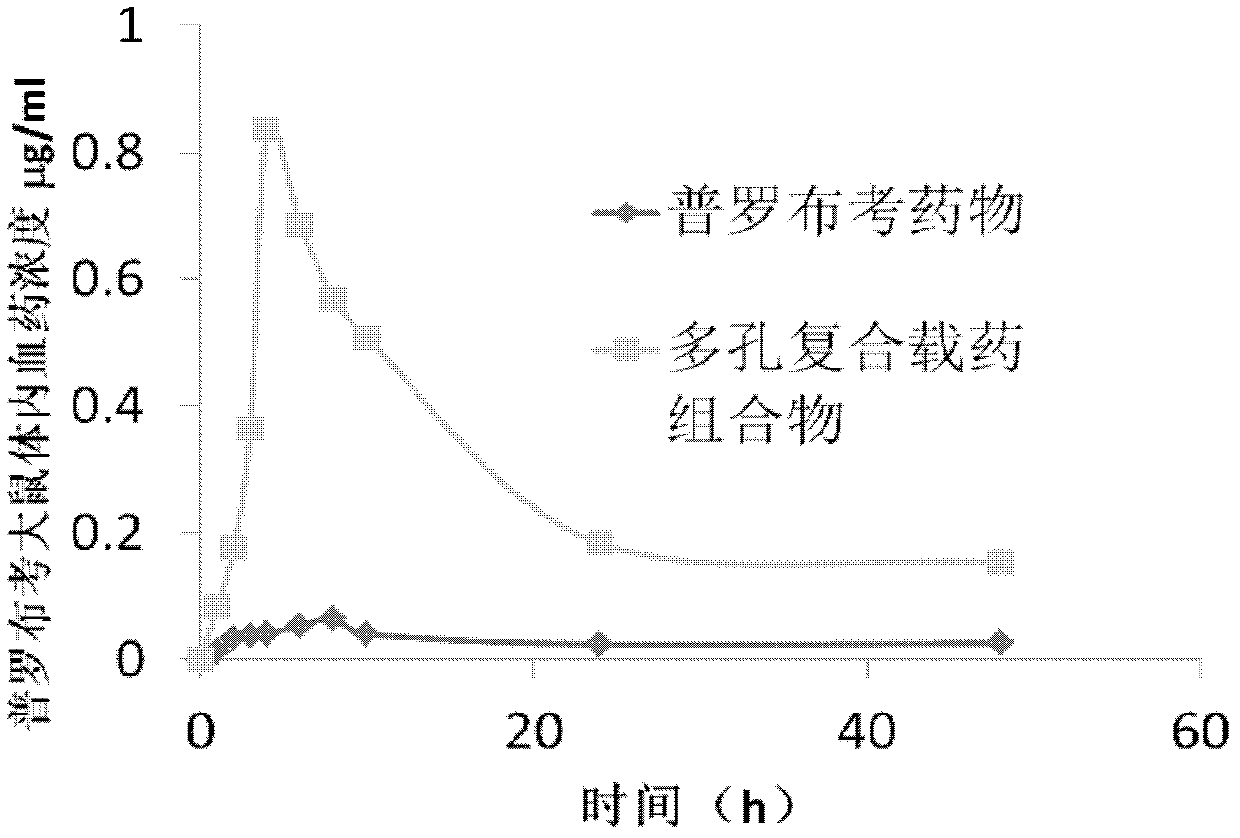
A technology of probucol and composition, applied in the field of medicine, can solve the problems of low oral bioavailability, large individual differences, and low bioavailability, and achieve improved oral bioavailability, thermodynamic stability, and high bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Preparation of probucol suspension: the original drug probucol was added to 0.5% hydroxypropyl cellulose aqueous solution and vortexed for 3 minutes to form.
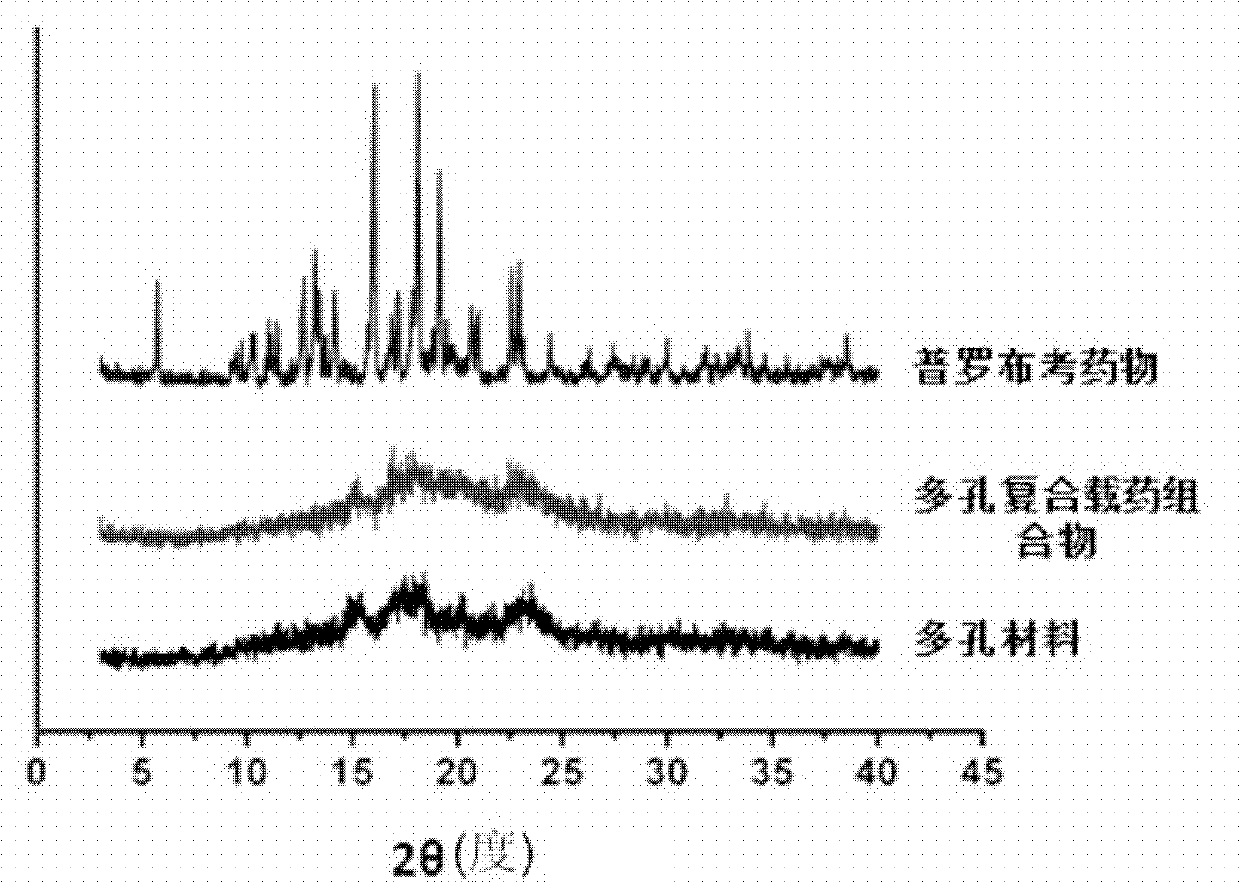
[0046] Preparation of porous calcium carbonate: porous calcium carbonate can be quickly mixed with the same volume of CaCl at room temperature 2 (Contains sodium dodecyl sulfate (SDS)) and Na 2 CO 3 Aqueous solution to prepare. First, under vigorous stirring, add the same volume of 0.2mol / L CaCl 2 The solution and 0.1moL / L SDS solution are mixed quickly, keep stirring and add 0.2mol / L Na equal to the volume of the mixed solution 2 CO 3 Solution. The precipitate was collected by centrifugation, washed three times with deionized water and absolute ethanol, and dried in an oven at 60°C. The sample was calcined at 350°C for 3 hours to remove the residual surfactant SDS in the sample. After calcination, porous calcium carbonate was obtained. CaCO prepared 3 , The hollow microspheres with a nanostructured porous shell, th...
Embodiment 1
[0052] (1) Test materials:
[0053] Probucol technical (Hebei Wuyi Cihang Pharmaceutical Co., Ltd., batch number 20100904), PVPP (American International Specialty Products Company, batch number: 03700178591), isopropyl myristate (Fluka company, batch number: 121617121106124), Cremophor RH40 (Germany BASF, batch number: 04770897V0), ethanol (Sinopharm Chemical Reagent Co., Ltd.).
[0054]
[0055] (2) Test method:
[0056] According to the steps of method two: accurately weigh 1.50 g of isopropyl myristate, Cremophor RH403.85 g, and 2.95 g of ethanol, mix well, weigh 1.00 g of probucol and dissolve in the above solution to obtain a clear solution. Separately weigh 17.50 g of PVPP and stir and mix with the clear solution to obtain a probucol porous composite drug-loading composition.
[0057] (3) Test results:
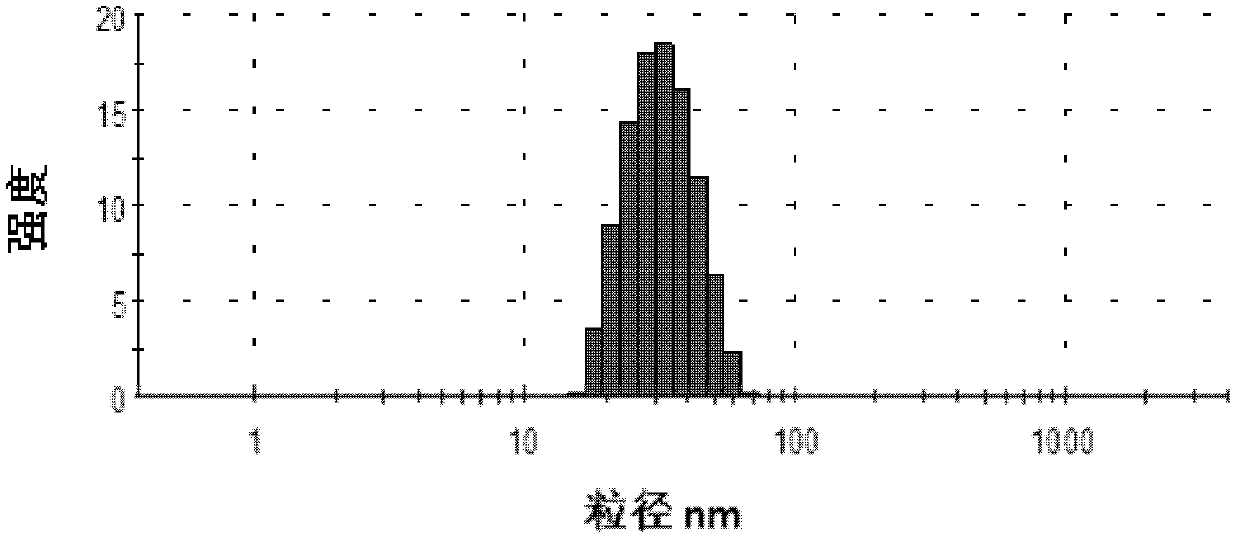
[0058] Measured by the Malvern nano zs90 (UK) particle size analyzer, the Z average particle size is 22.67nm, and the PDI is 0.239. Compared with probucol bulk drug, the solubi...
Embodiment 2
[0060] (1) Test materials:
[0061] Probucol technical (Hebei Wuyi Cihang Pharmaceutical Co., Ltd., batch number 20100904), diatomaceous earth (Sinopharm Group Chemical Reagent Co., Ltd., batch number 09050204), soybean oil (Tieling Beiya Pharmaceutical Oil Co., Ltd., batch number 09050204), spit Wen 80 (Sinopharm Group Chemical Reagent Co., Ltd., batch number F20100517), Propylene Glycol (Sinopharm Group Chemical Reagent Co., Ltd.).
[0062]
[0063]
[0064] (2) Test method:
[0065] According to the steps of method two: accurately weigh 0.50 g of soybean oil, 6.90 g of Tween, and 4.50 g of propylene glycol, mix well, weigh 1.00 g of probucol and dissolve in the above solution to obtain a clear solution. Separately weigh 19.50 g of diatomaceous earth and grind and mix with a clear solution to obtain a probucol porous composite drug-carrying composition.
[0066] (3) Test results:
[0067] Measured by the Malvern nano zs90 (UK) particle size analyzer, the Z average particle size is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com