Synthesis method of probucol
A synthesis method, the technology of probucol, applied in the field of synthesis of raw material probucol, can solve the problems of no product yield and purity, inaccurate yield data, low product purity, etc., to reduce product cost and high product purity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
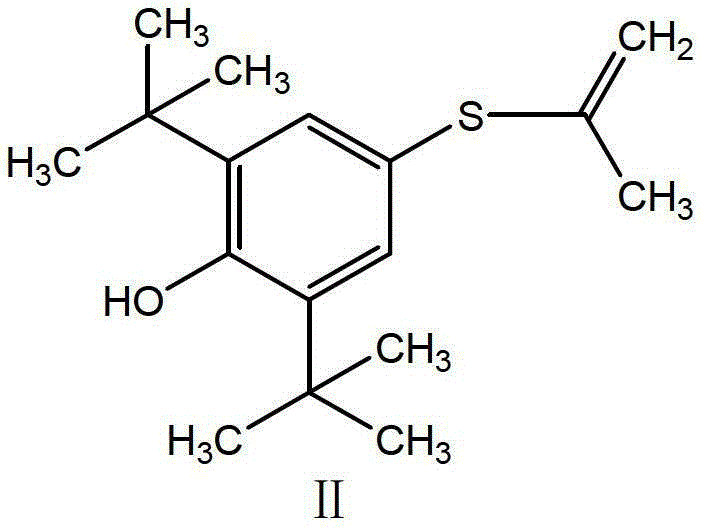
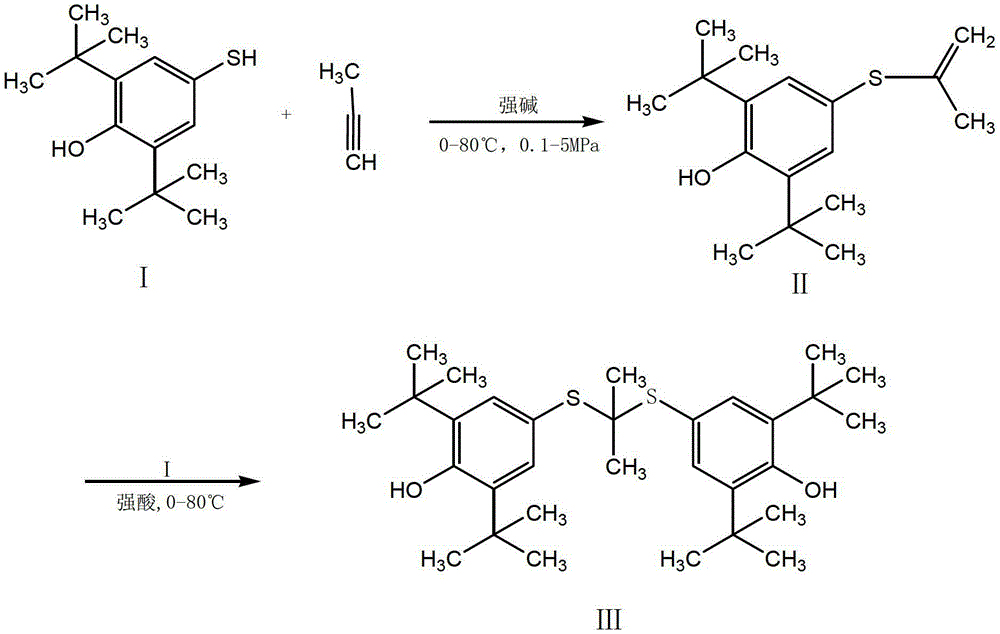
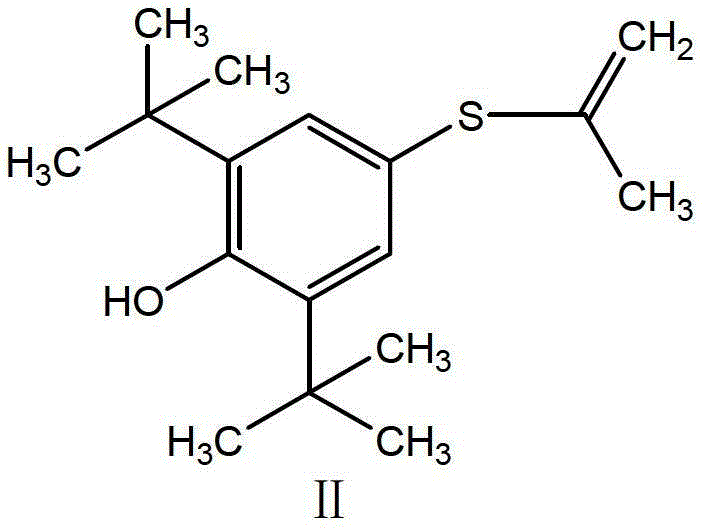
[0031] Raw material 2,6-di-tert-butyl-4-mercaptophenol solid (I) 47.7g (0.2mol), put it into a 0.5L autoclave, then add 76g methanol and 21.6g (0.40mol) sodium methoxide, heat Stir to dissolve at 50°C, continue heating to 60°C, pass in propyne, keep the pressure of 1MPa, stir and react for 3h, then release the pressure, transfer the liquid to a 500ml five-necked flask, add 2,6-di-tert 47.7g (0.2mol) of butyl-4-mercaptophenol, add 35% hydrochloric acid by mass concentration under stirring to make pH≤1, heat to 60-65℃, keep for 1h, then cool to 5-15℃, stir for 4-6h, filter with suction , The crude probucol was obtained, washed with water, then refined with 95% ethanol, and dried in vacuum to obtain 96.9 g of the pure probucol, with a total yield of 93.8% and an HPLC purity of 99.90%. mp125~126℃.
[0032] Product element analysis (%): the measured value C is 71.96, H is 9.44, S is 12.36; the theoretical value C is 72.04, H is 9.36, and S is 12.41. IR (KBr) cm-1: 3634, 3078, 2960, 2...
Embodiment 2
[0034] As described in Example 1, the difference is that the volume of the reactor used is doubled, and the influence of the amount of alkyne on the reaction is investigated.
[0035] Add 47.7g (0.2mol) of 2,6-di-tert-butyl-4-mercaptophenol into a 1.0L autoclave, add 76g of methanol and 21.6g (0.4mol) of sodium methoxide, heat to 50℃ and stir to dissolve the raw materials , Continue to heat to 60 ℃, pass in propyne, and maintain the pressure of 1 MPa, stir the reaction for 3 hours and release the pressure, transfer to a 500ml five-necked flask, and then add 2,6-di-tert-butyl-4-mercaptophenol 47.7 g(0.2mol), heat to 60℃ and stir to dissolve, add 35% hydrochloric acid by mass concentration to adjust pH=0.8, continue to heat to 60-65℃, keep for 1h, then cool to 5-15℃, stir for 4-6h, filter by suction and wash with water Then it was refined with 95% ethanol and dried in vacuum to obtain 97.6 g of probucol. The total yield was 94.5% and the HPLC purity was 99.93%.
Embodiment 3
[0037] As described in Example 2, the difference is that 110 g of ethanol is used instead of 76 g of methanol in Example 2, and 26.4 g (0.4 mol) of sodium ethoxide is used instead of sodium methoxide in Example 2. Finally, 94.0 g of probucol was obtained, the total yield was 90.9%, and the HPLC purity was 99.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com