Pharmaceutical compositions for minocycline
a technology of compositions and minocycline, which is applied in the field of compositions comprising minocycline, can solve the problems of reduced compliance rate of subjects, wide variations, and subjects experiencing vestibular adverse effects, and achieve the effect of predicting and accurate fractional doses of minocyclin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-3
[0320]The tablets of the present application comprising minocycline are prepared as described herein.
TABLE 1Quantity per unit (%)CompositionEx-1Ex - 2Ex - 3Ex - 4Drug loadingInert core14.861312.6613Minocycline Hydrochloride15.812.6713.4612.67(equivalent to Minocycline 135mg)Hydroxy propyl cellulose3.453.453.453.45Hydroxy propyl methyl cellulose1.801.851.851.85Polyethylene glycol 4000.520.690.670.69Talc0.791.821.771.82Water qs to 15.7% w / wqsqsqsqsTotal37.2233.5333.7133.53Barrier coatingHydroxy propyl methyl cellulose1.861.671.681.67Polyethylene glycol 4000.180.160.160.16Talc0.550.500.500.50WaterqsqsqsqsTotal2.592.342.352.34Extended release coatingEthyl cellulose5.434.693.934.69Hydroxy propyl methyl cellulose1.811.561.311.56Triethyl citrate0.540.460.390.46Talc2.171.871.571.87Isopropyl alcoholqsqsqsqsWaterqsqsqsqsTotal9.958.607.28.60Outer top coatingHydroxy propyl methyl cellulose5.343.803.863.80Polyethylene glycol 4000.530.380.380.38Talc1.601.131.161.13WaterqsqsqsqsTotal7.475.325.45.3...
example 5
[0331]The tablet comprising minocycline as prepared in example 4 was subjected to content uniformity studies as intact tablet and after dividing into two equal subunits. The results are shown in table 2.
TABLE 2IntactAssay % - Example 4No. of unitsIntact tabletDivision 1Division 2110199952991029539710193497103935961039369810193799999289899919999994109910292Mean98.510193Acceptance3.848.7Value% Relative1.511.661.44standarddeviationResultPassPassPass
example 6
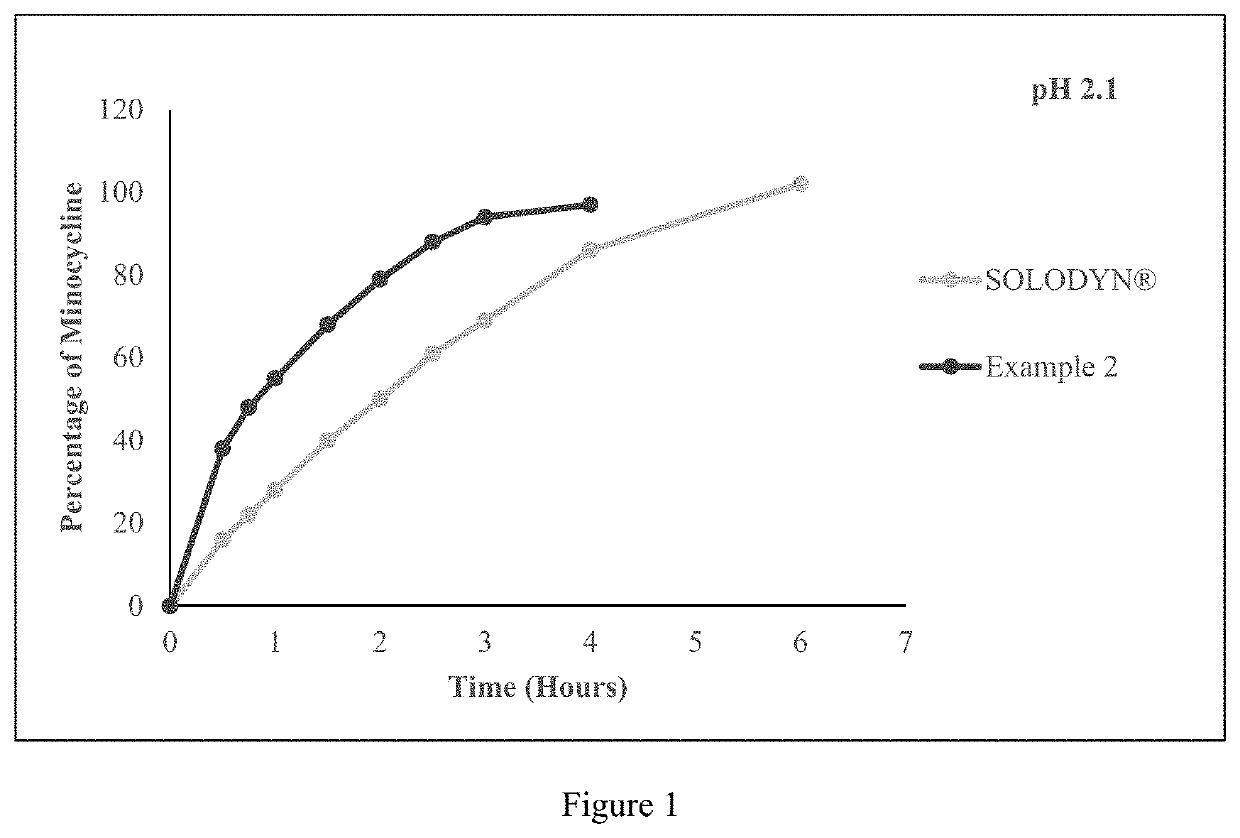
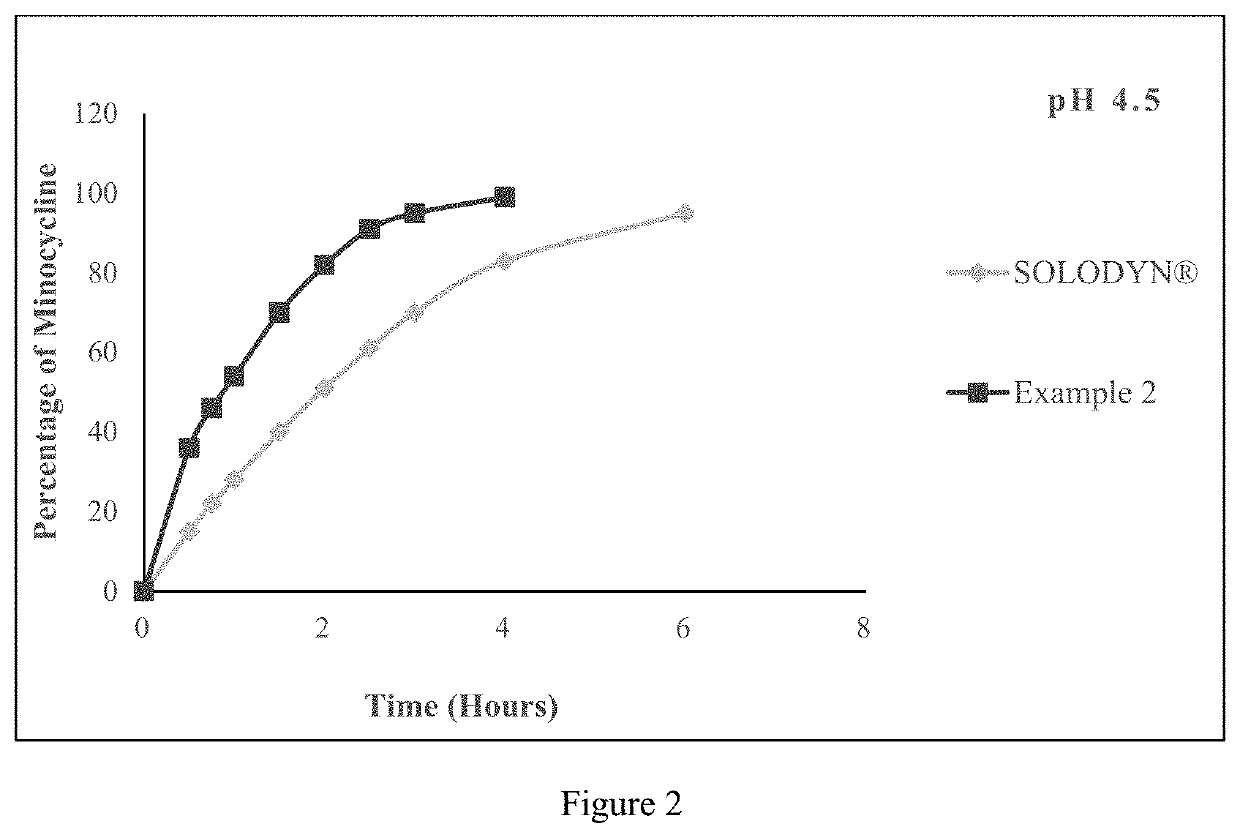
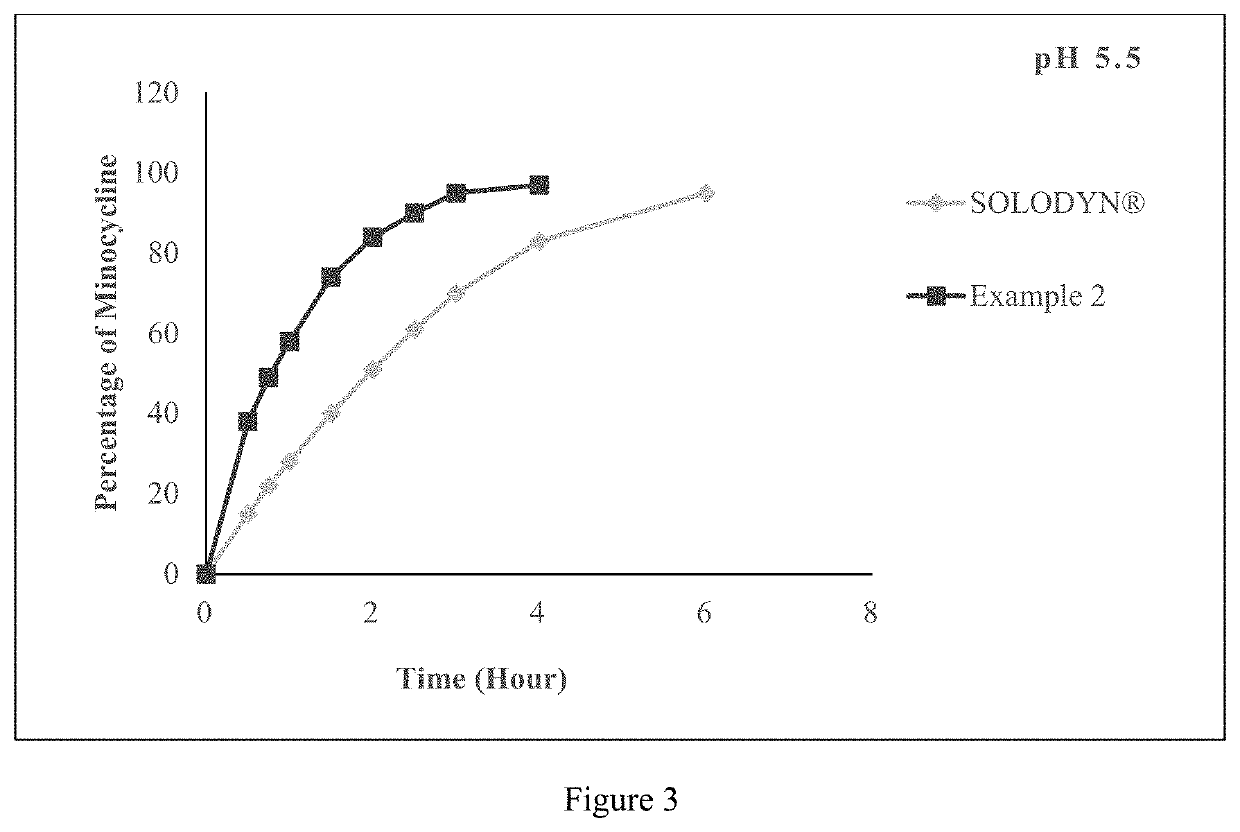
[0332]The tablet comprising minocycline as prepared in example 2 was subjected to dissolution studies in 900 ml of pH 2.1 Simulated Gastric Fluid, pH 4.5 acetate buffer and pH 5.5 phosphate buffer with USP Type I apparatus at a speed of 100 rpm and 37° C. till 6 hours and the in-vitro release profile are shown in FIGS. 1, 2 and 3 respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com