Patents
Literature
47 results about "Transplant patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
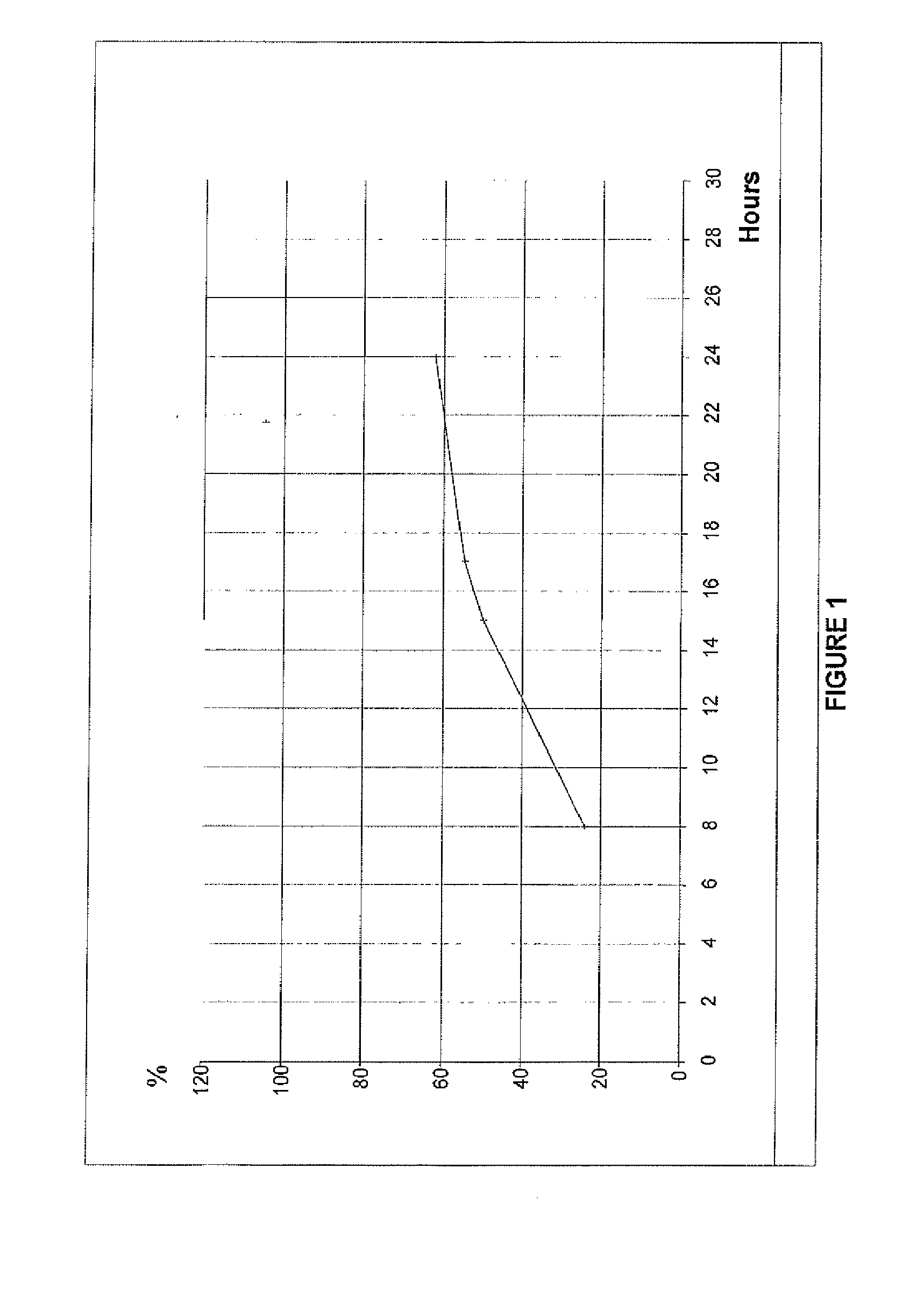
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
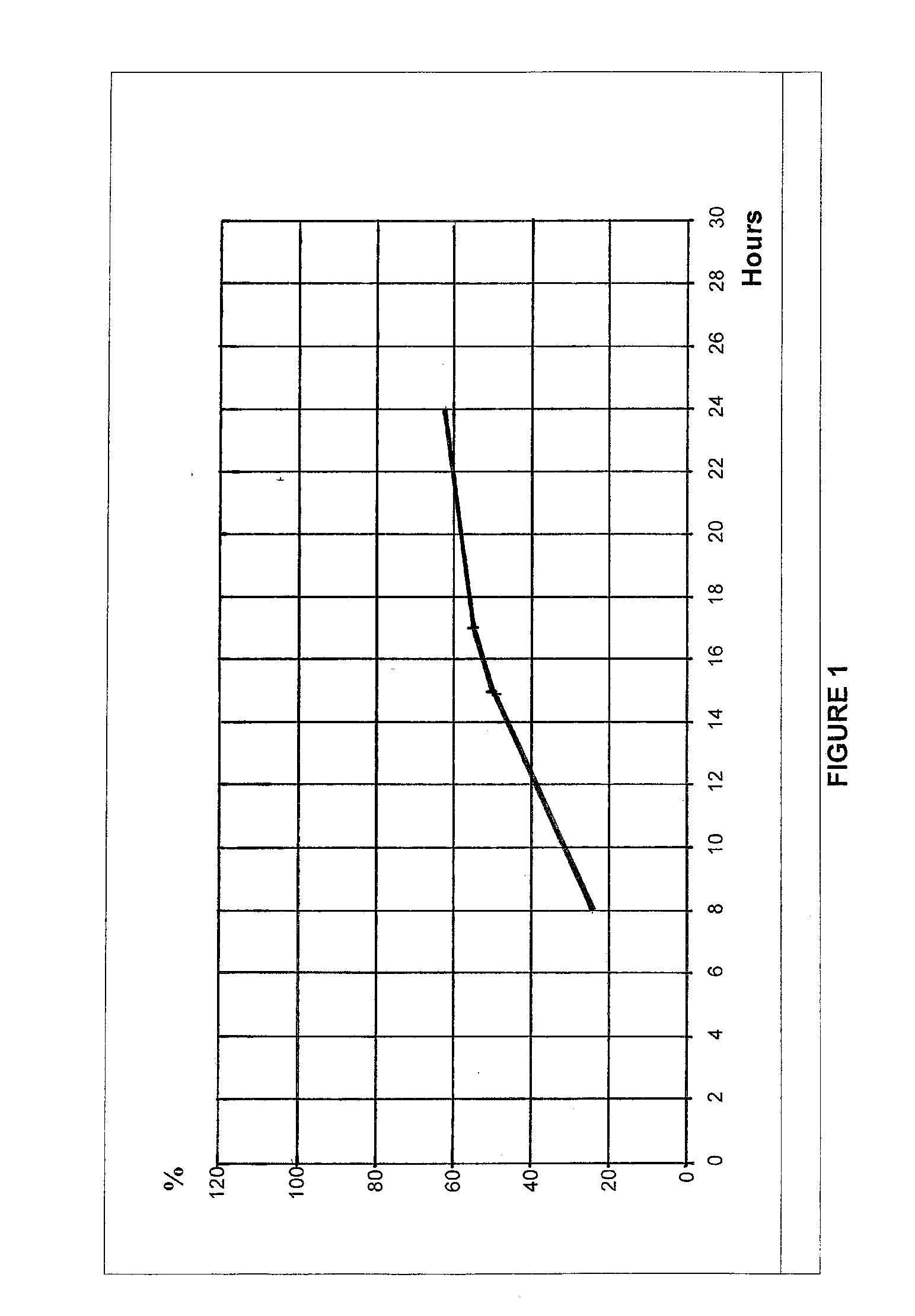
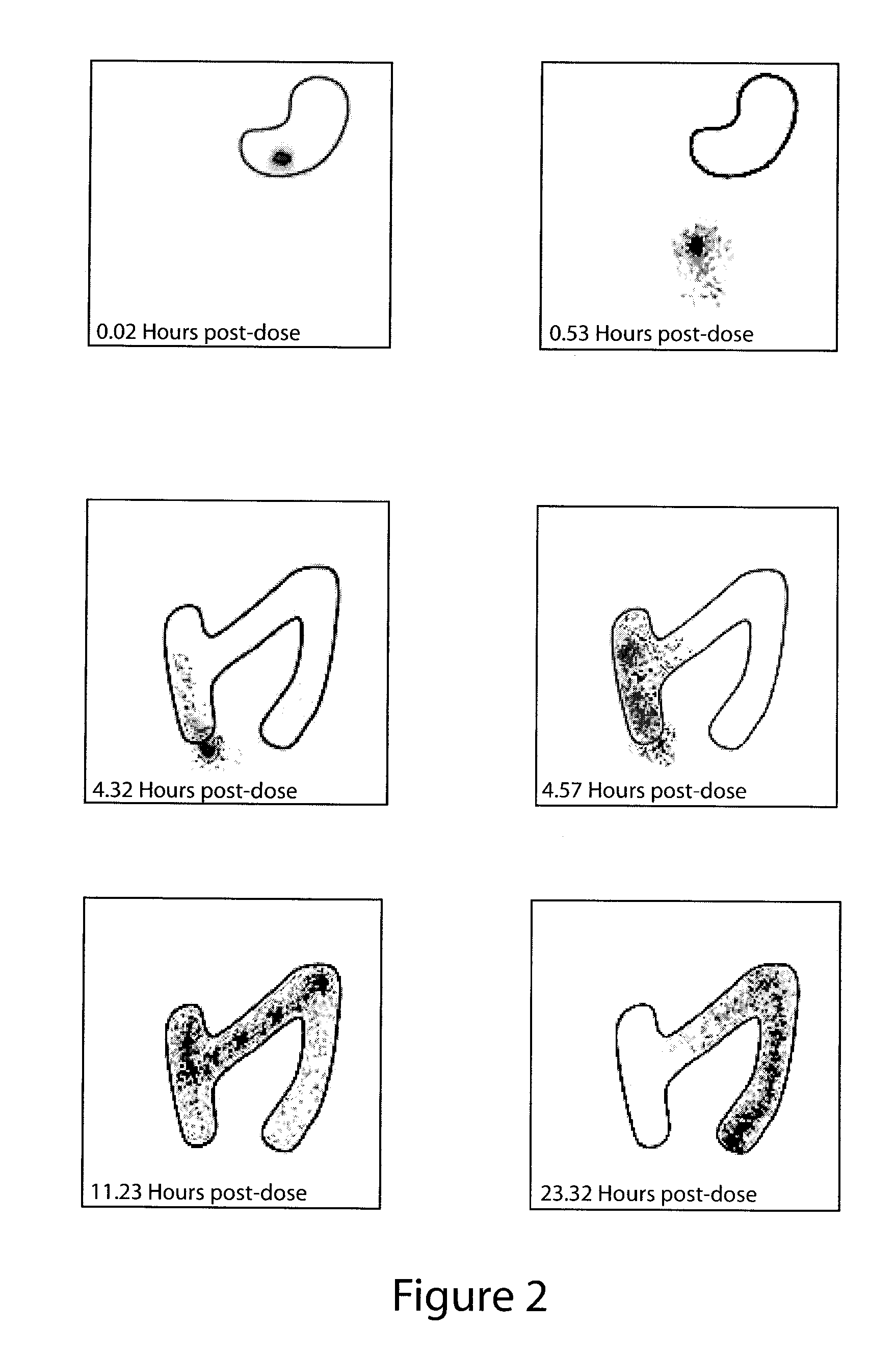
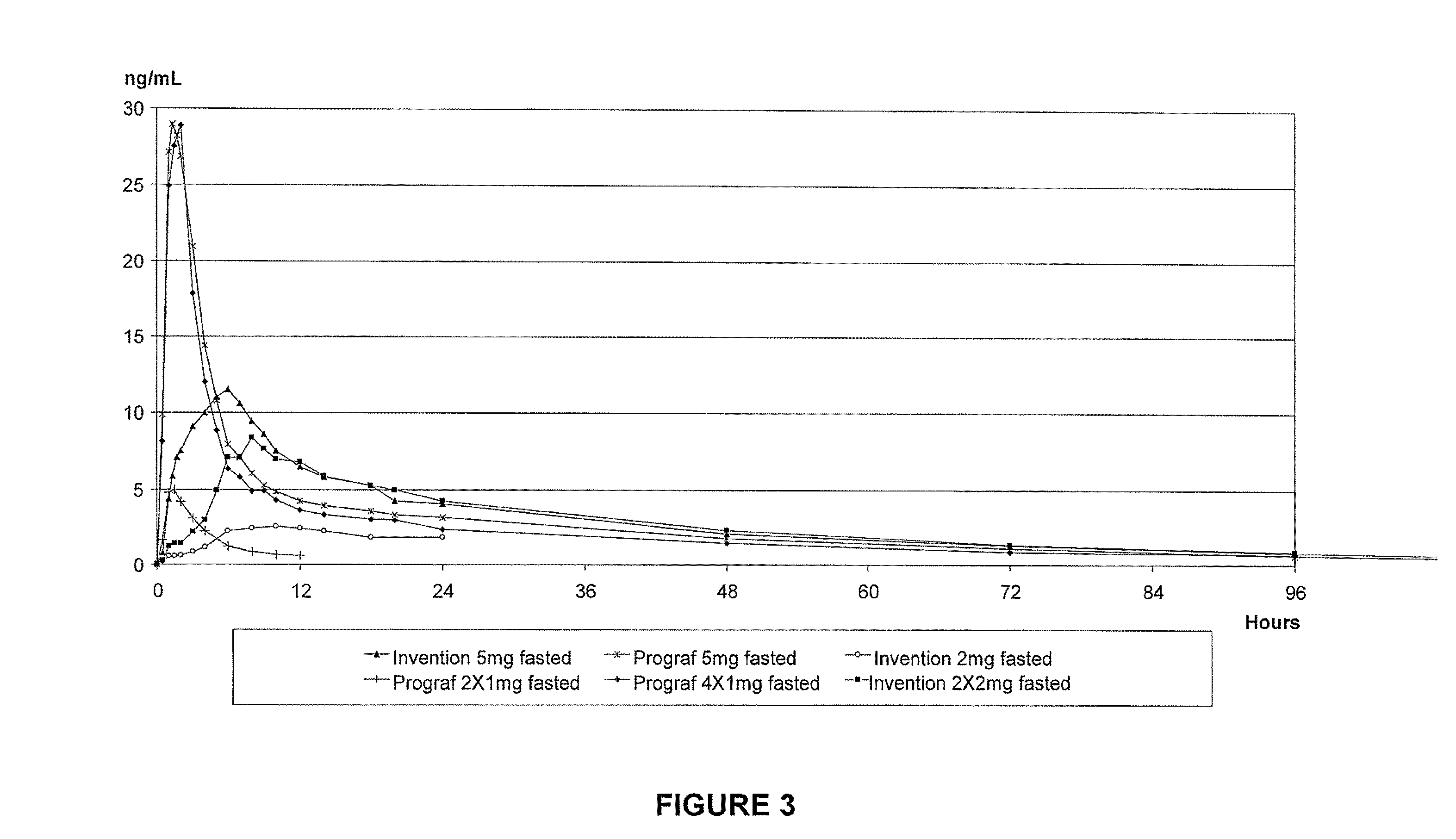
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
Reduction in bacterial colonization by administering bacteriophage compositions
InactiveUS7459272B2Reduce and eliminate colonizationReduce riskAntibacterial agentsBiocideBacteroidesAcquired immunodeficiency
The present invention provides a method for reducing the risk of bacterial infection or sepsis in a susceptible patient by treating the susceptible patient with a pharmaceutical composition containing bacteriophage of one or more strains which produce lytic infections in pathogenic bacteria. Preferably, treatment of the patient reduces the level of colonization with pathogenic bacteria susceptible to the bacteriophage by at least one log. In a typical embodiment, the susceptible patient is an immunocompromised patient selected from the group consisting of leukemia patients, lymphoma patients, carcinoma patients, sarcoma patients, allogeneic transplant patients, congenital or acquired immunodeficiency patients, cystic fibrosis patients, and AIDS patients. In a preferred mode, the patients treated by this method are colonized with the pathogenic bacteria subject to infection by said bacteriophage.
Owner:INTRALYTIX
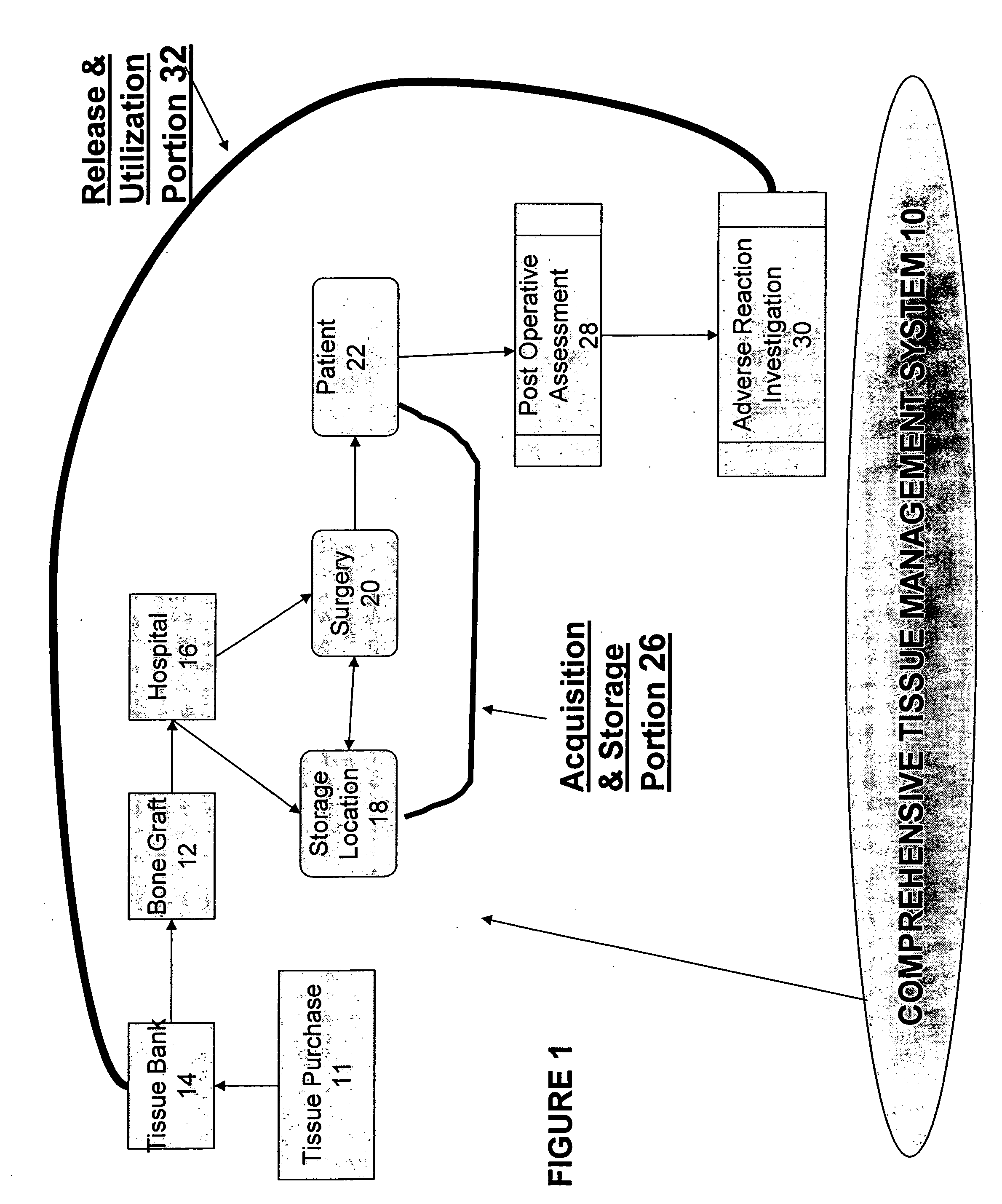
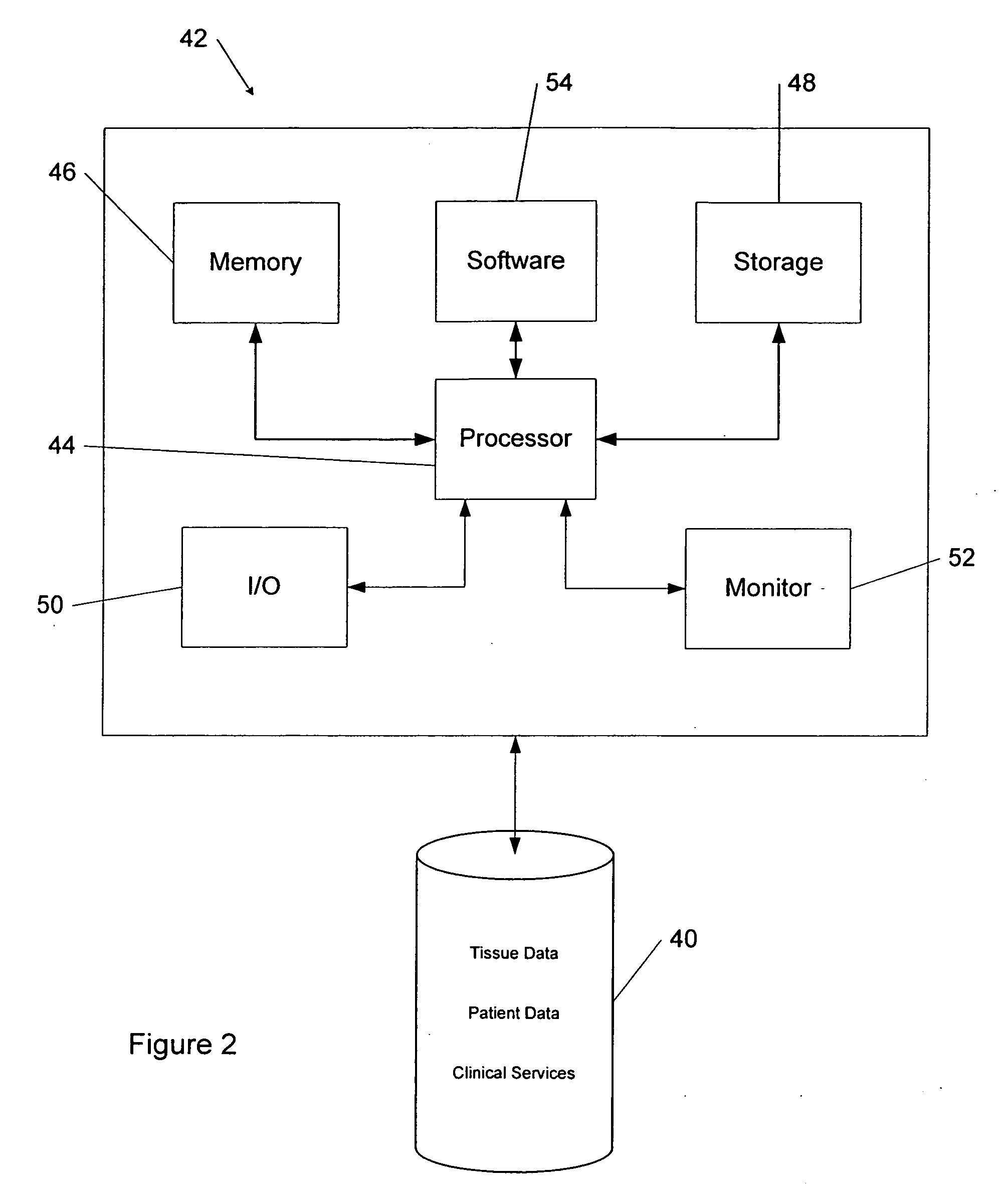
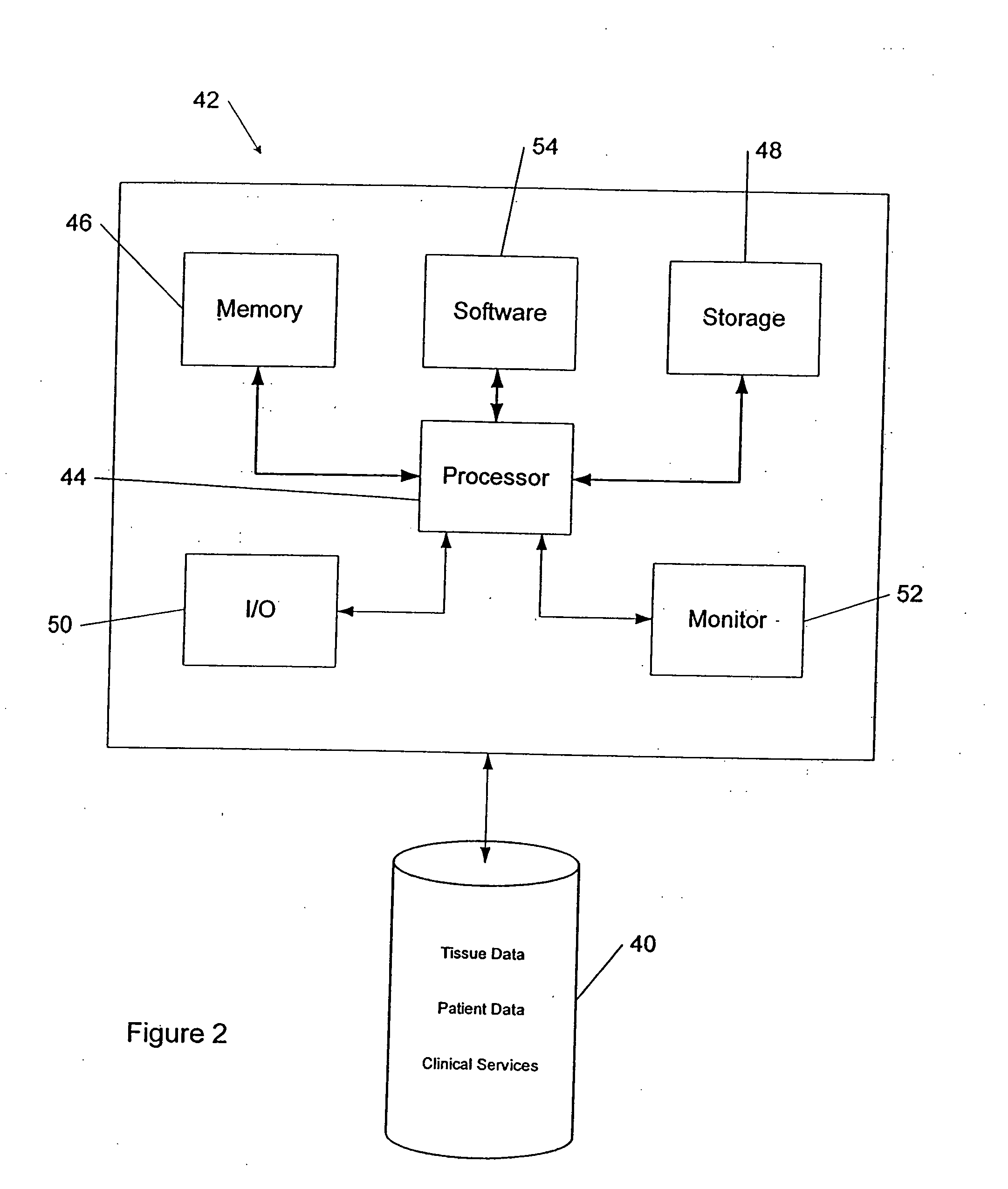
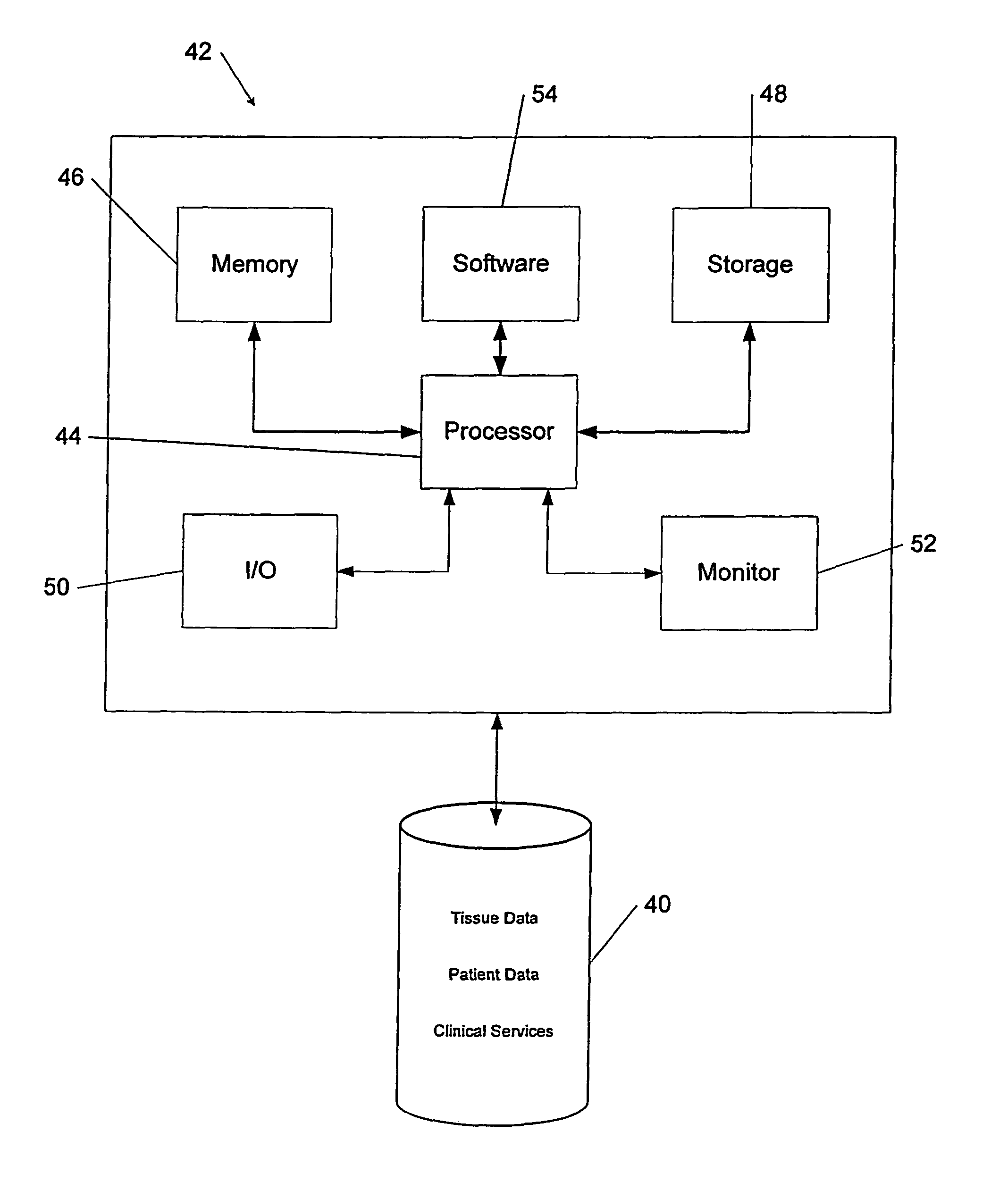
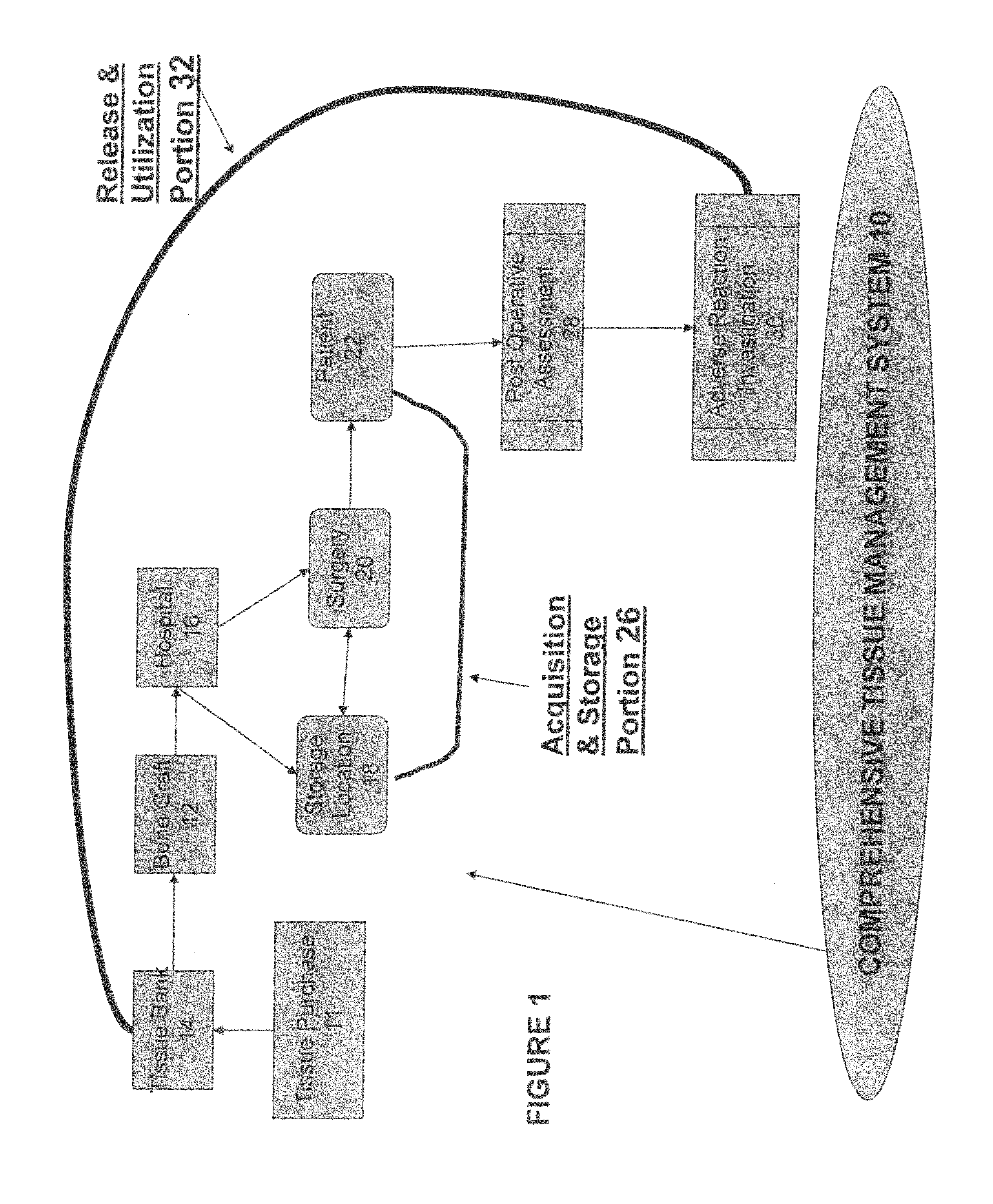
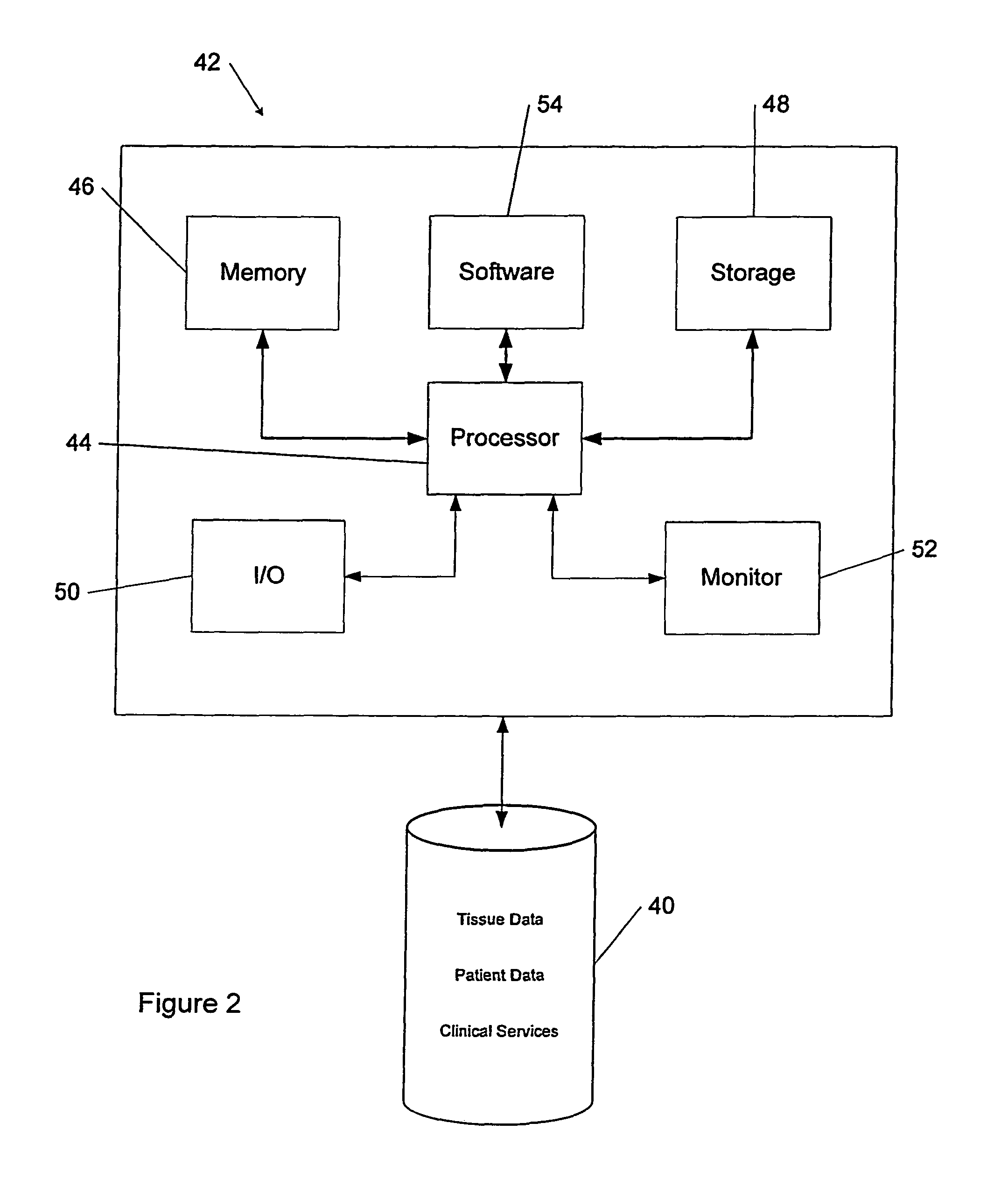
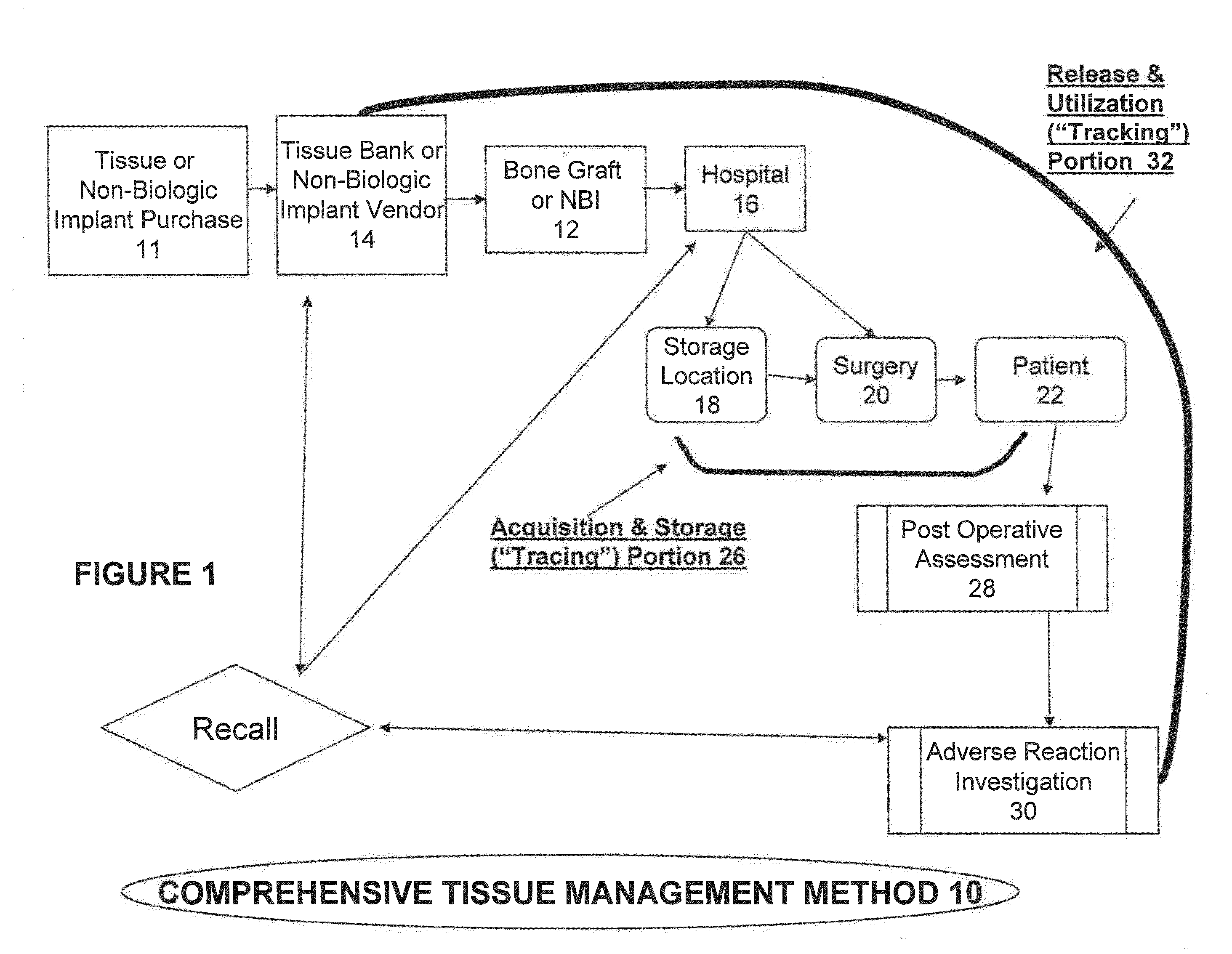
Tissue management system
ActiveUS20080077433A1Computer-assisted medical data acquisitionDiagnostic recording/measuringMedical institutionManagement system
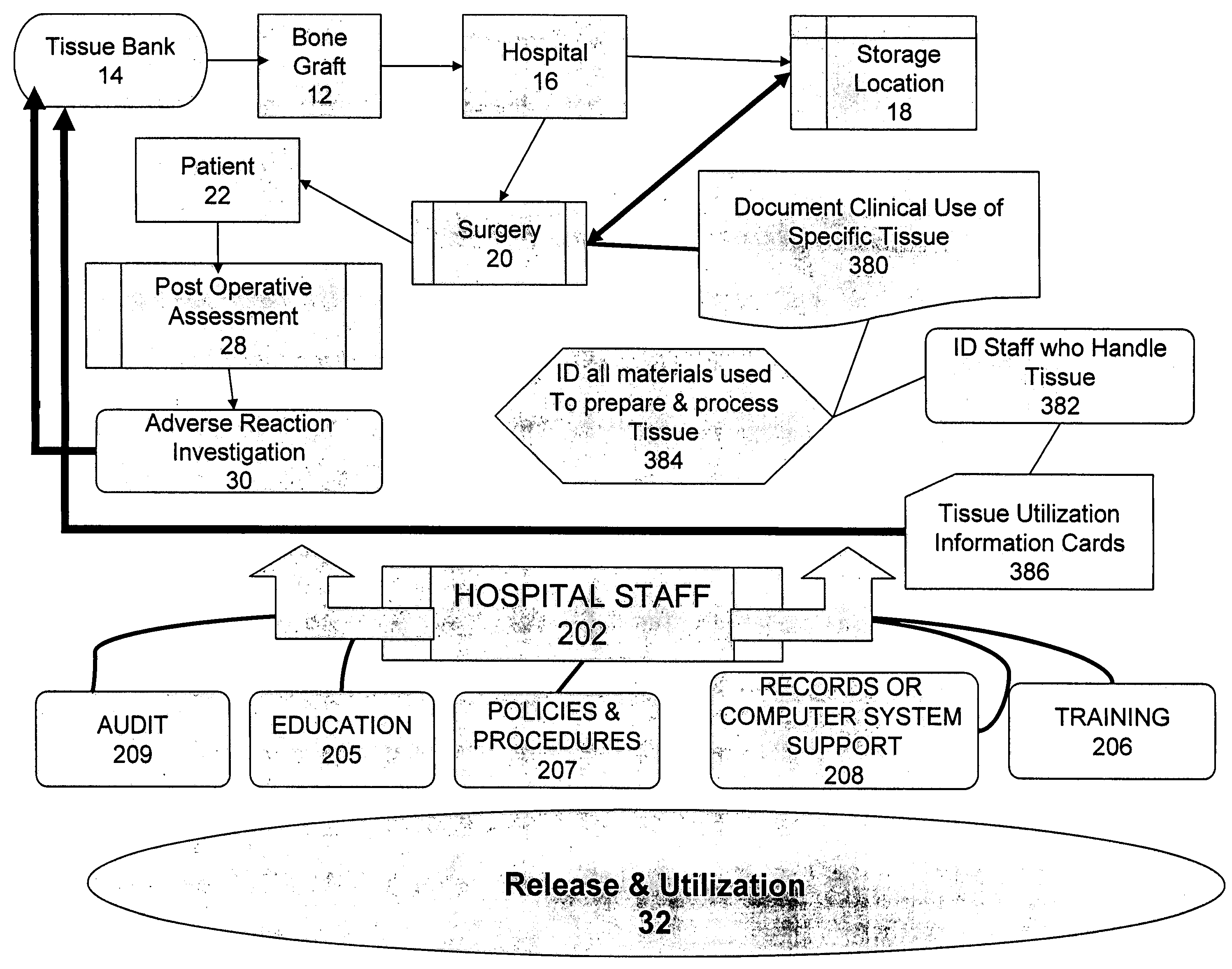
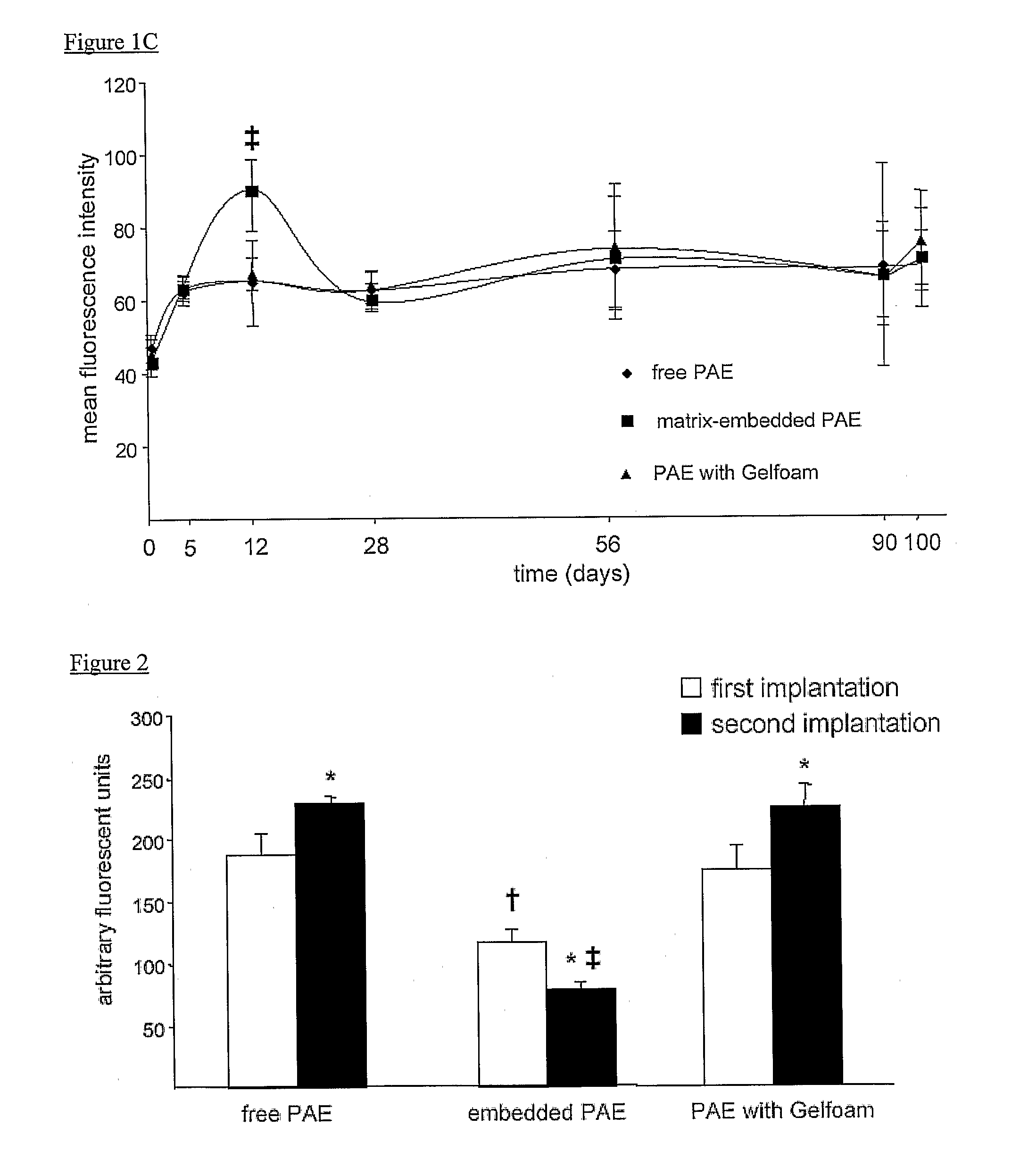
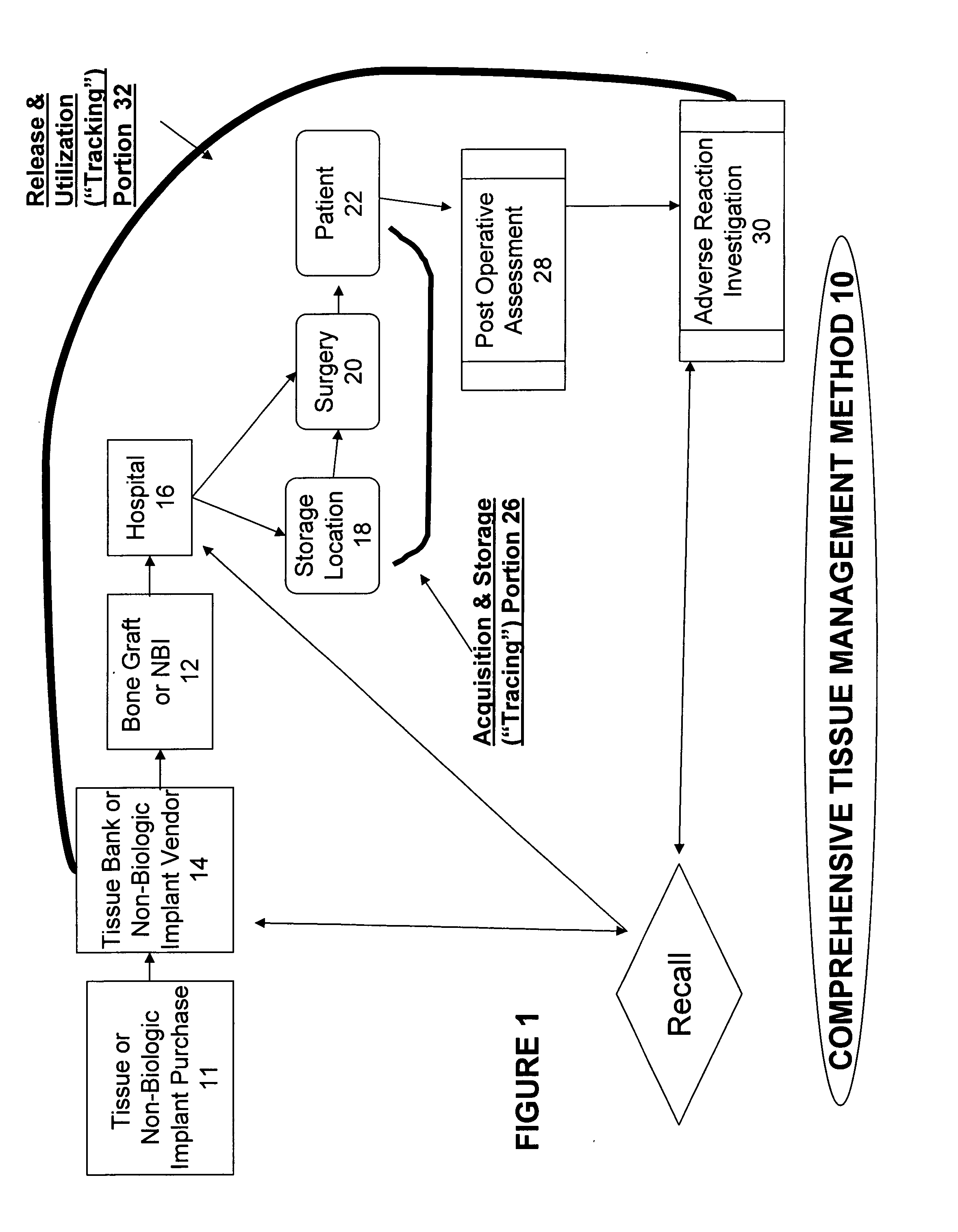
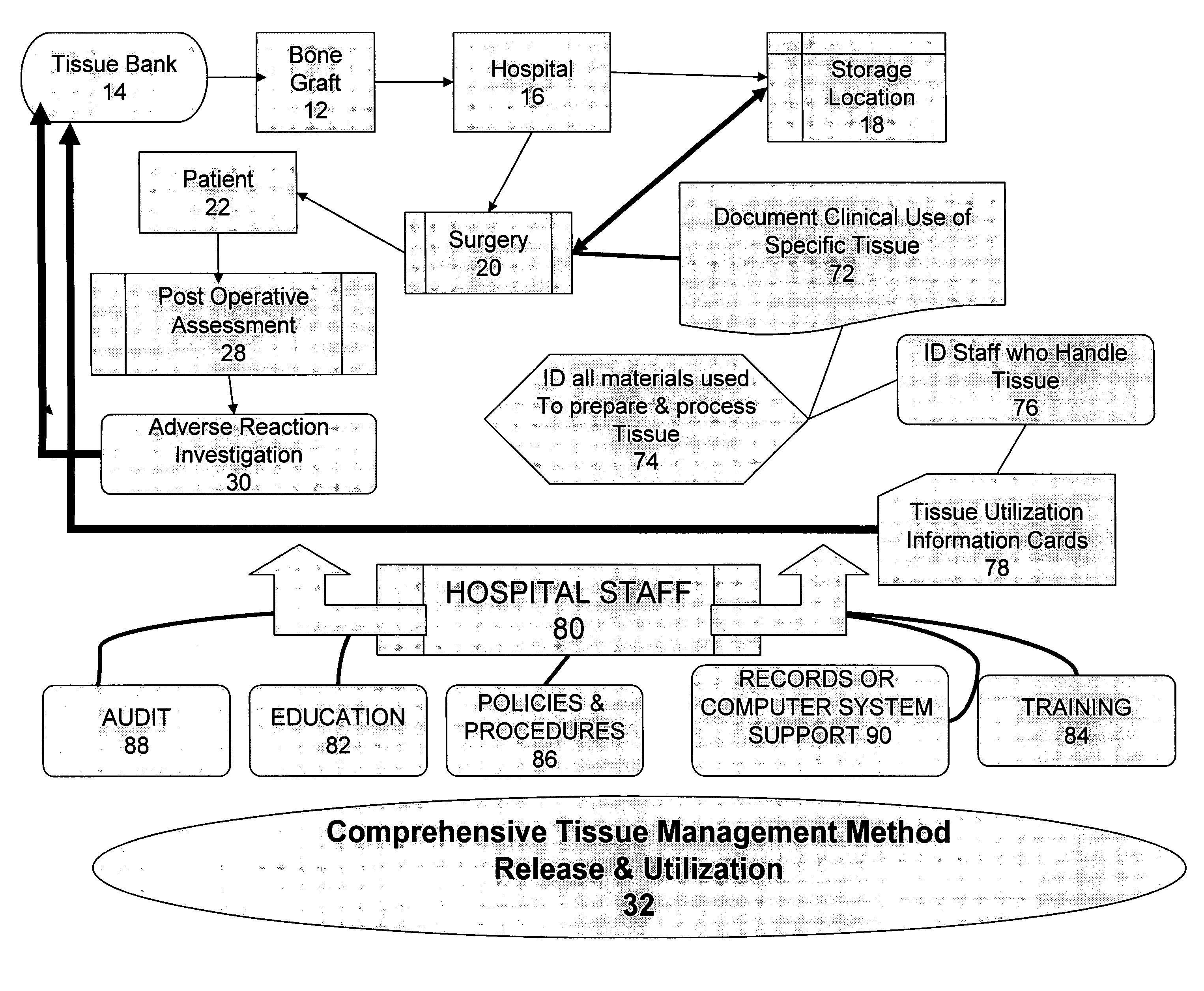
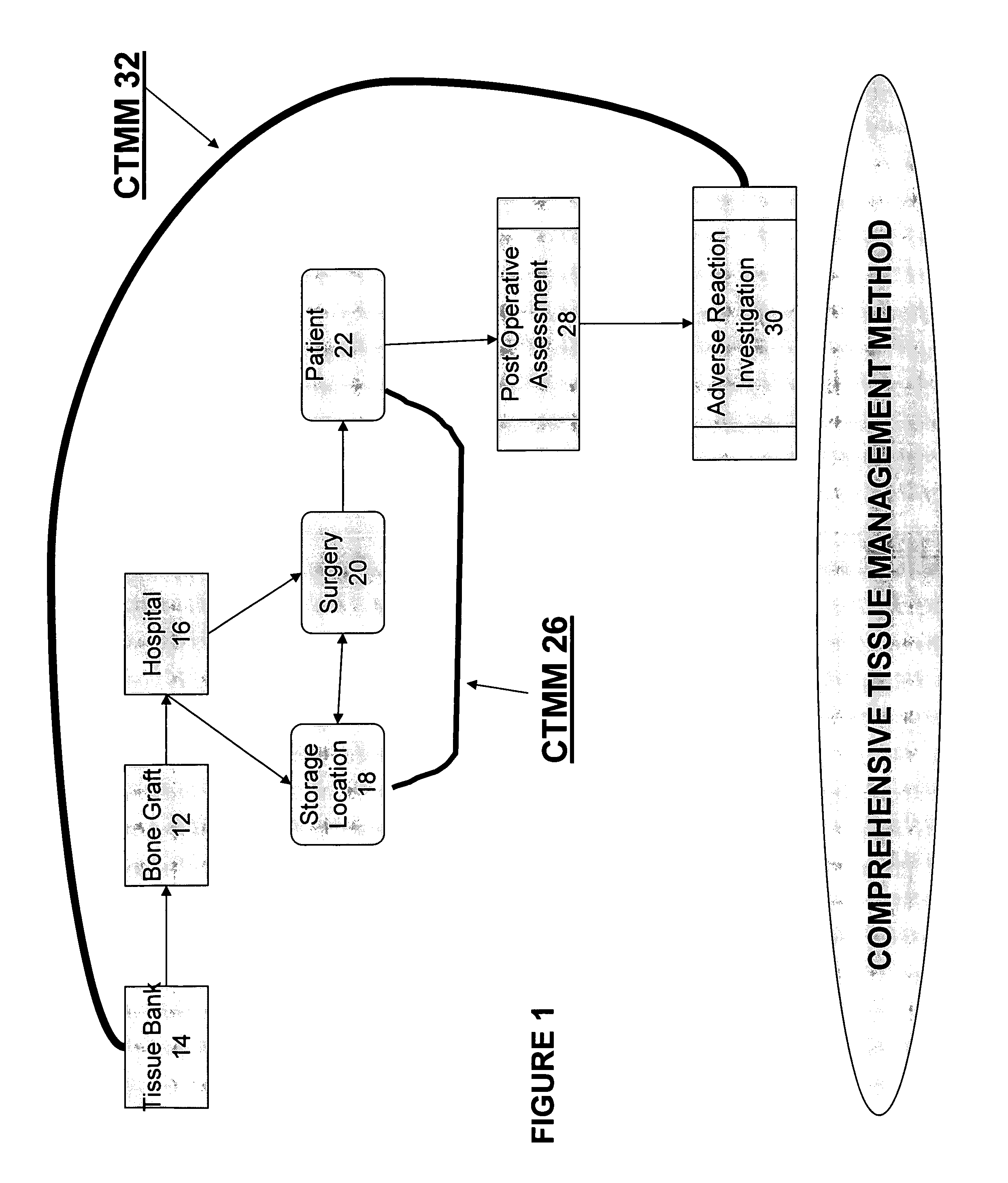
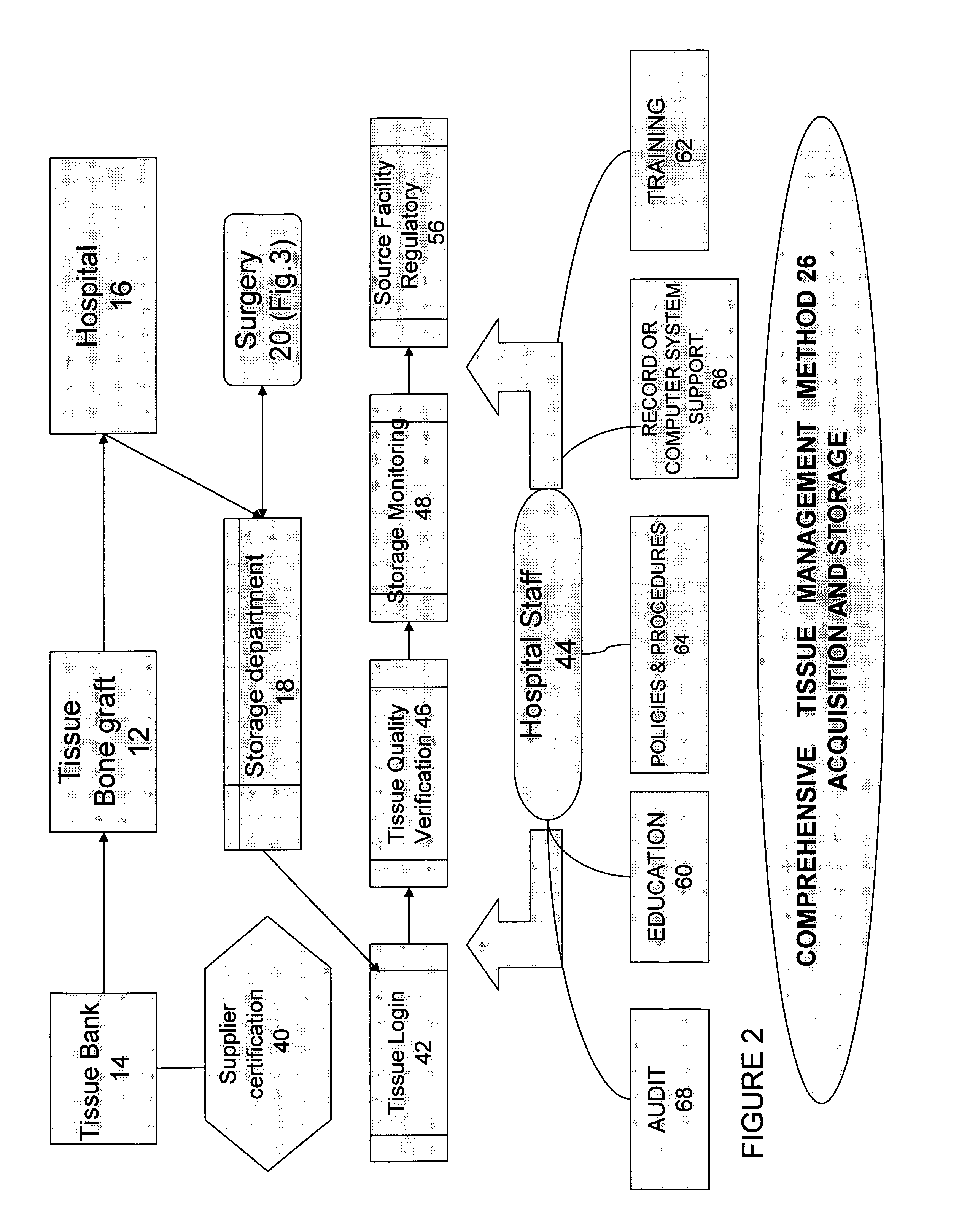
The present invention provides a comprehensive tissue management system for transplantable materials like tissues and organs. The tracking portion of the system prompts and verifies that staff members of a medical establishment like a hospital have handled, stored, transported, reconstituted, and used the tissue or organ materials in a safe and regulatory-compliant manner from the point of receipt to the point of issuance or surgical use throughout the hospital's organization. The tracing portion of the system creates an integral record that documents which hospital staff members have provided which processing steps to the tissue or organ, any associated materials used in conjunction with such tissue or organ, and an identification of the tissue or organ that was transplanted or implanted inside a patient. Such a system will enable adverse reaction investigations for transplant patients, and recalls of transplantable materials.
Owner:BIOMEDICAL SYNERGIES
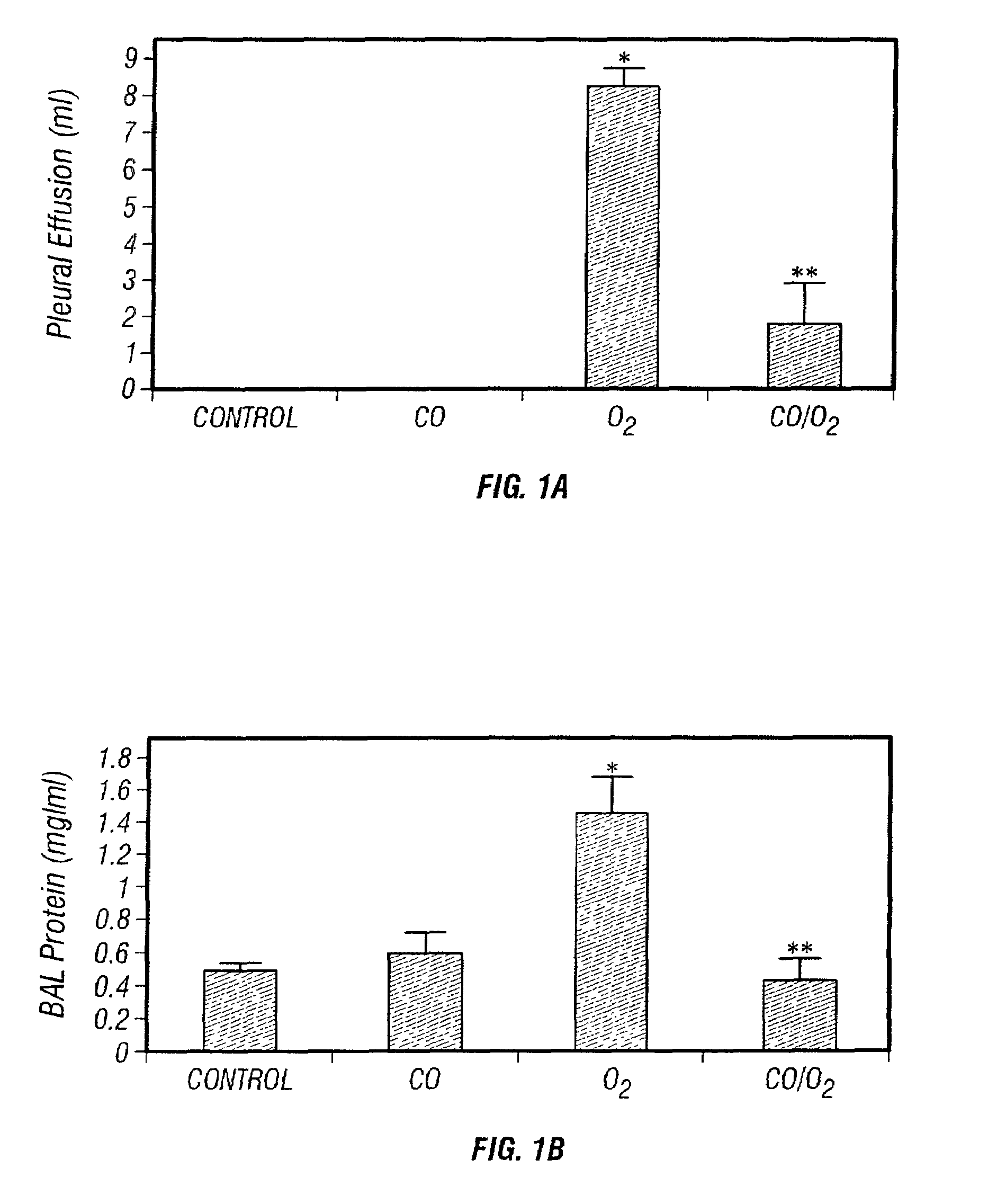
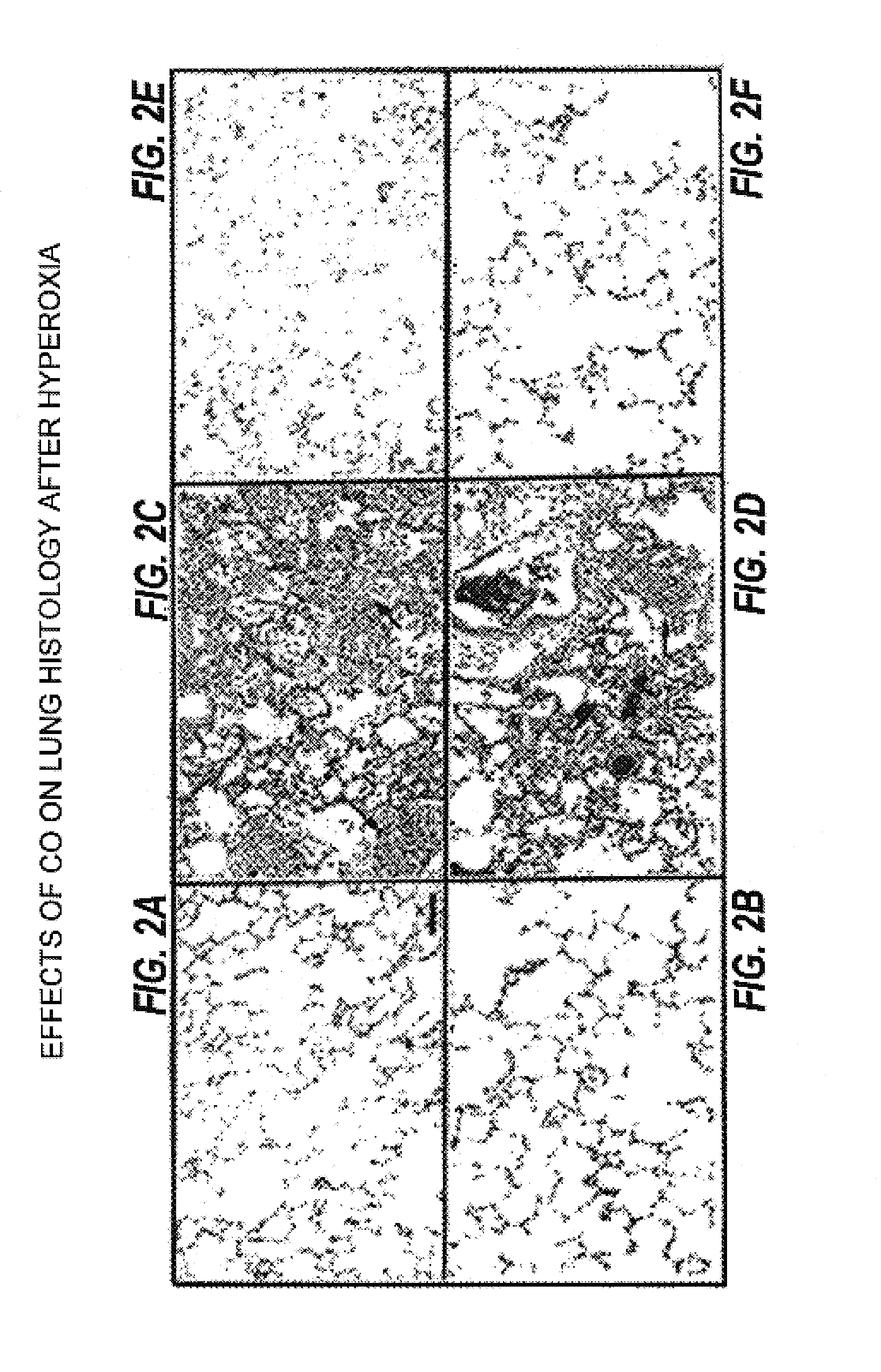
Carbon monoxide as a biomarker and therapeutic agent
InactiveUS7678390B2Reduce concentrationInhibiting and alleviating effectBiocideInorganic active ingredientsRESPIRATORY DISTRESS SYNDROME ADULTInterstitial lung disease
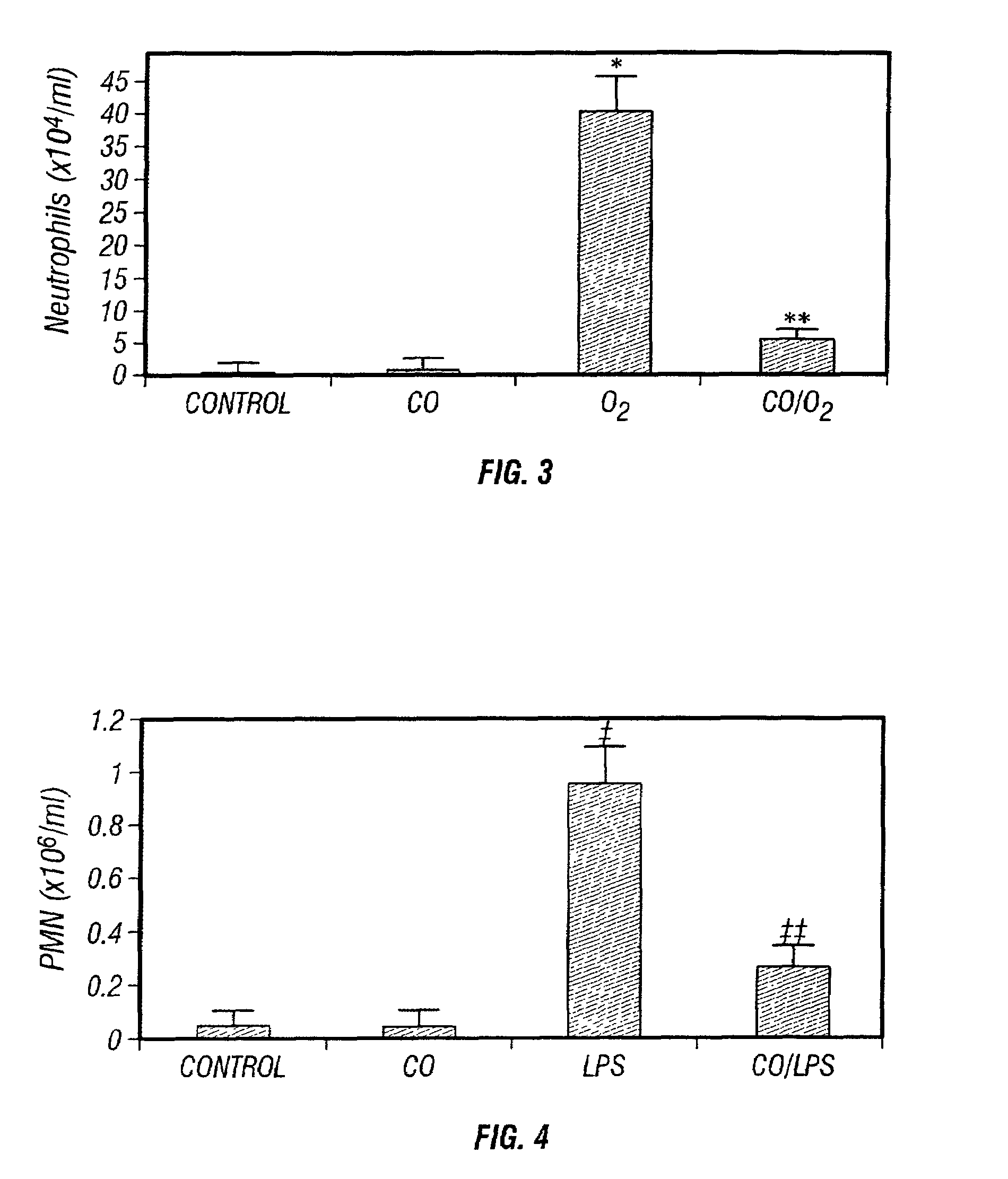
The present invention relates to the use of carbon monoxide (CO) as a biomarker and therapeutic agent of heart, lung, liver, spleen, brain, skin and kidney diseases and other conditions and disease states including, for example, asthma, emphysema, bronchitis, adult respiratory distress syndrome, sepsis, cystic fibrosis, pneumonia, interstitial lung diseases, idiopathic pulmonary diseases, other lung diseases including primary pulmonary hypertension, secondary pulmonary hypertension, cancers, including lung, larynx and throat cancer, arthritis, wound healing, Parkinson's disease, Alzheimer's disease, peripheral vascular disease and pulmonary vascular thrombotic diseases such as pulmonary embolism. CO may be used to provide anti-inflammatory relief in patients suffering from oxidative stress and other conditions especially including sepsis and septic shock. In addition, carbon monoxide may be used as a biomarker or therapeutic agent for reducing respiratory distress in lung transplant patients and to reduce or inhibit oxidative stress and inflammation in transplant patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Materials and Methods for Altering an Immune Response to Exogenous and Endogenous Immunogens, Including Syngeneic and Non-Syngeneic Cells, Tissues or Organs
InactiveUS20120308610A1Reduce reduction reactionReduced responseAntipyreticAnalgesicsAutoimmune conditionGraft rejections
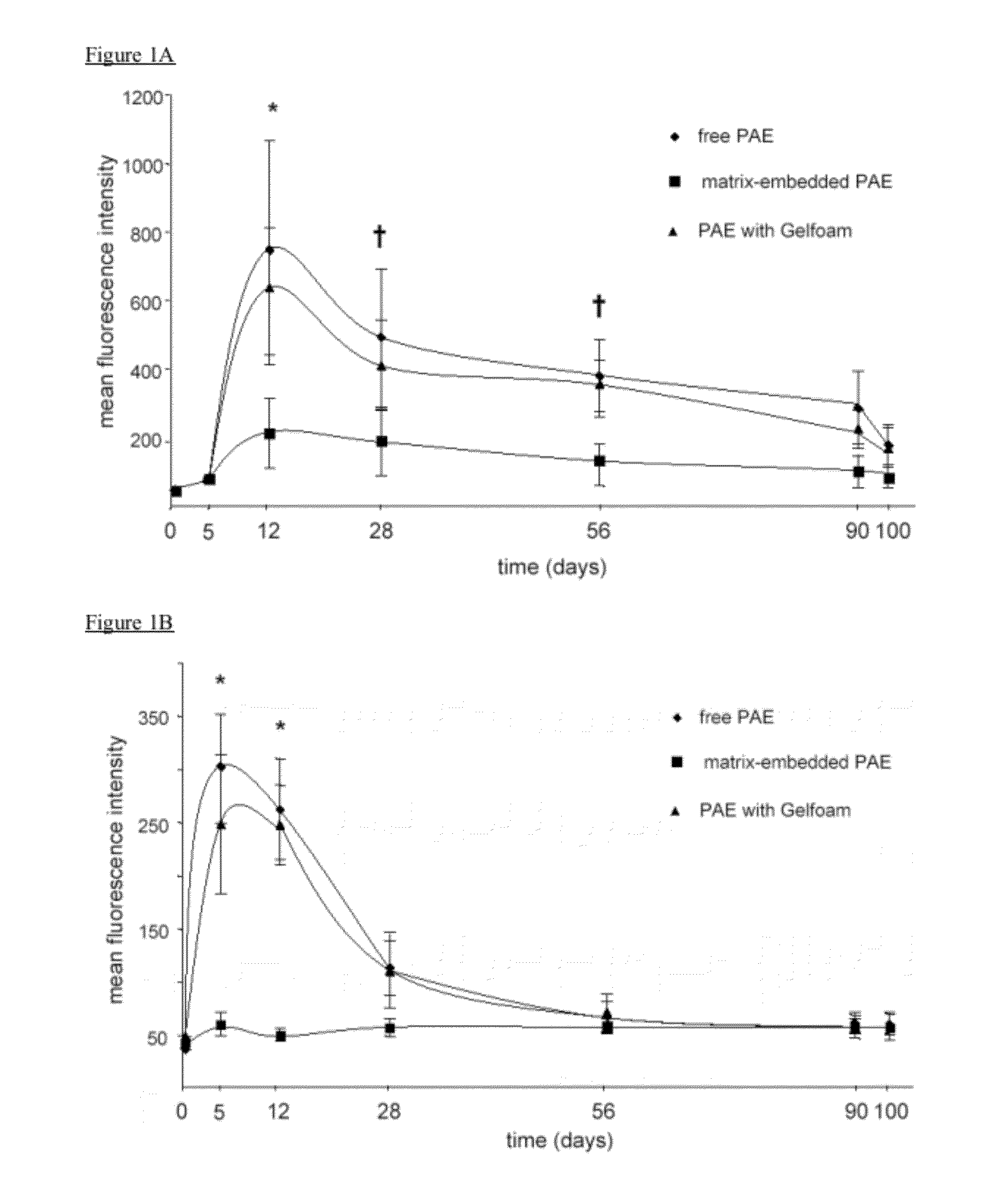
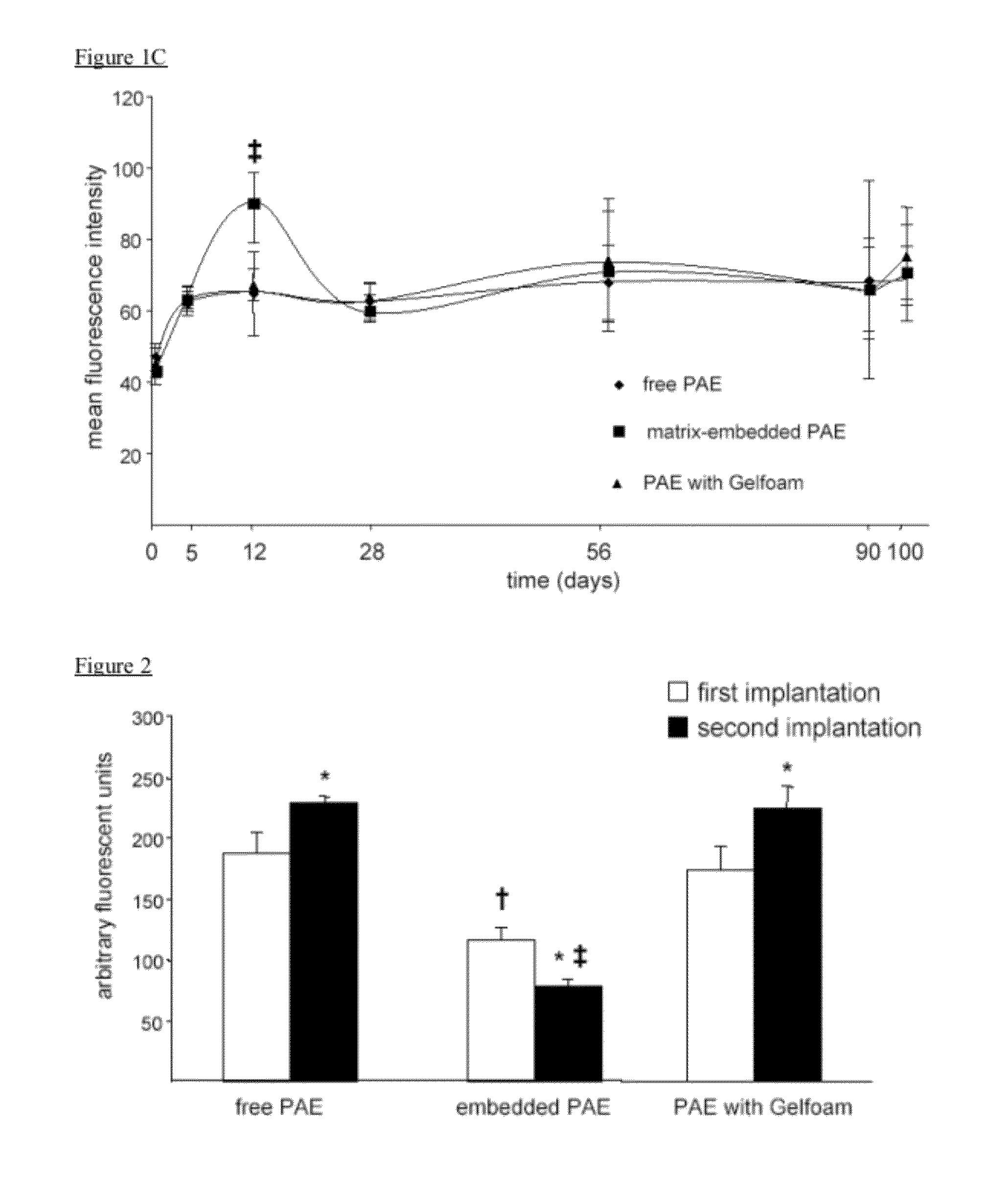
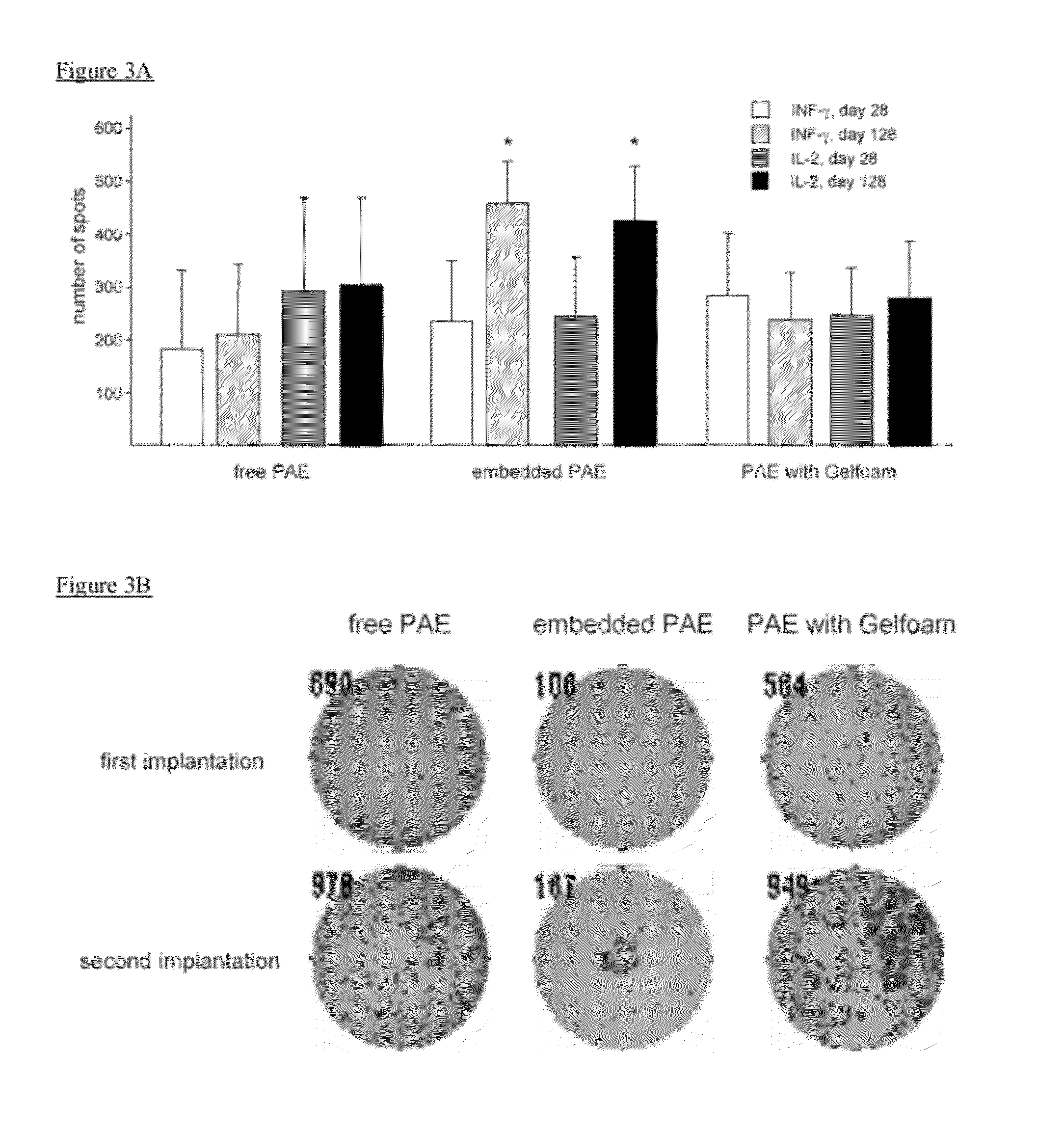
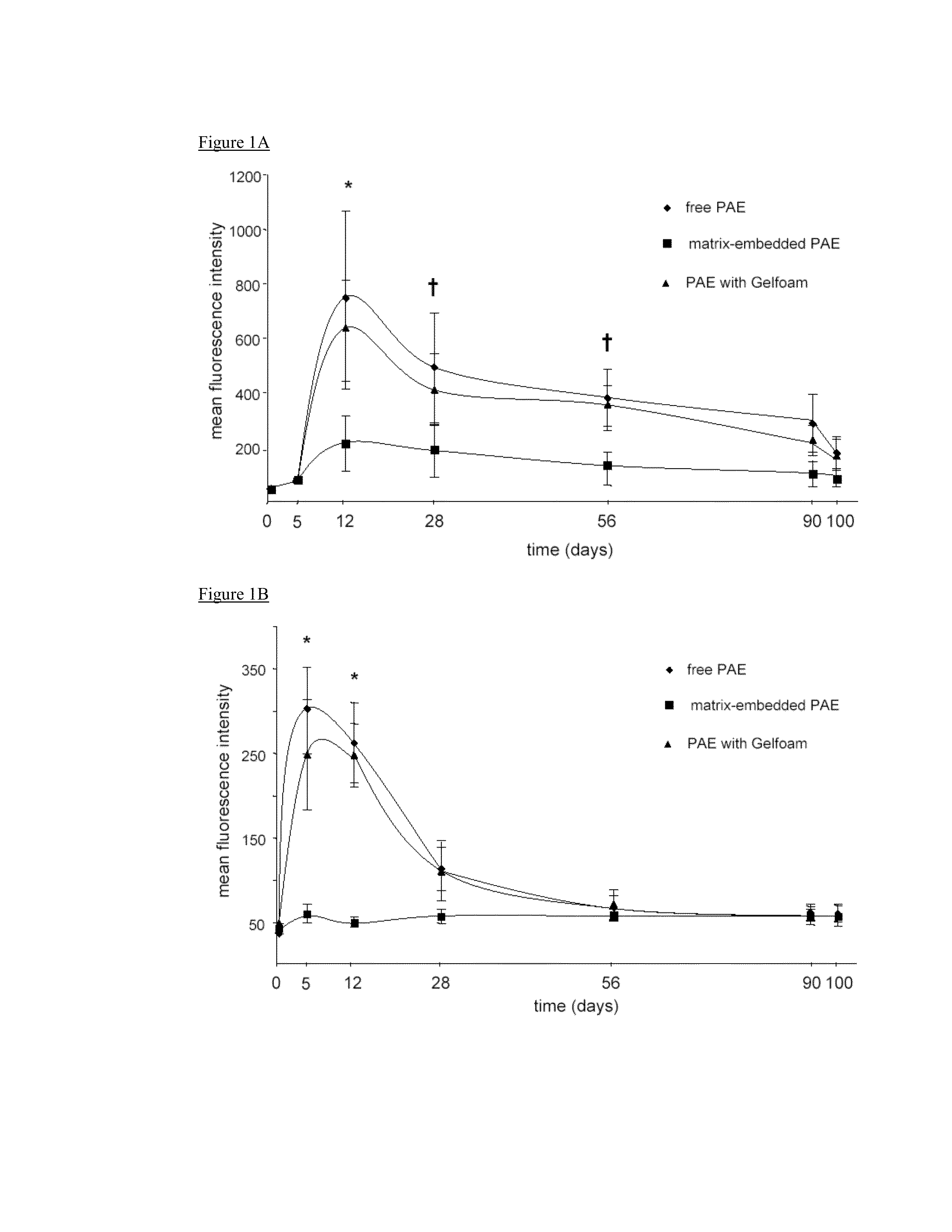
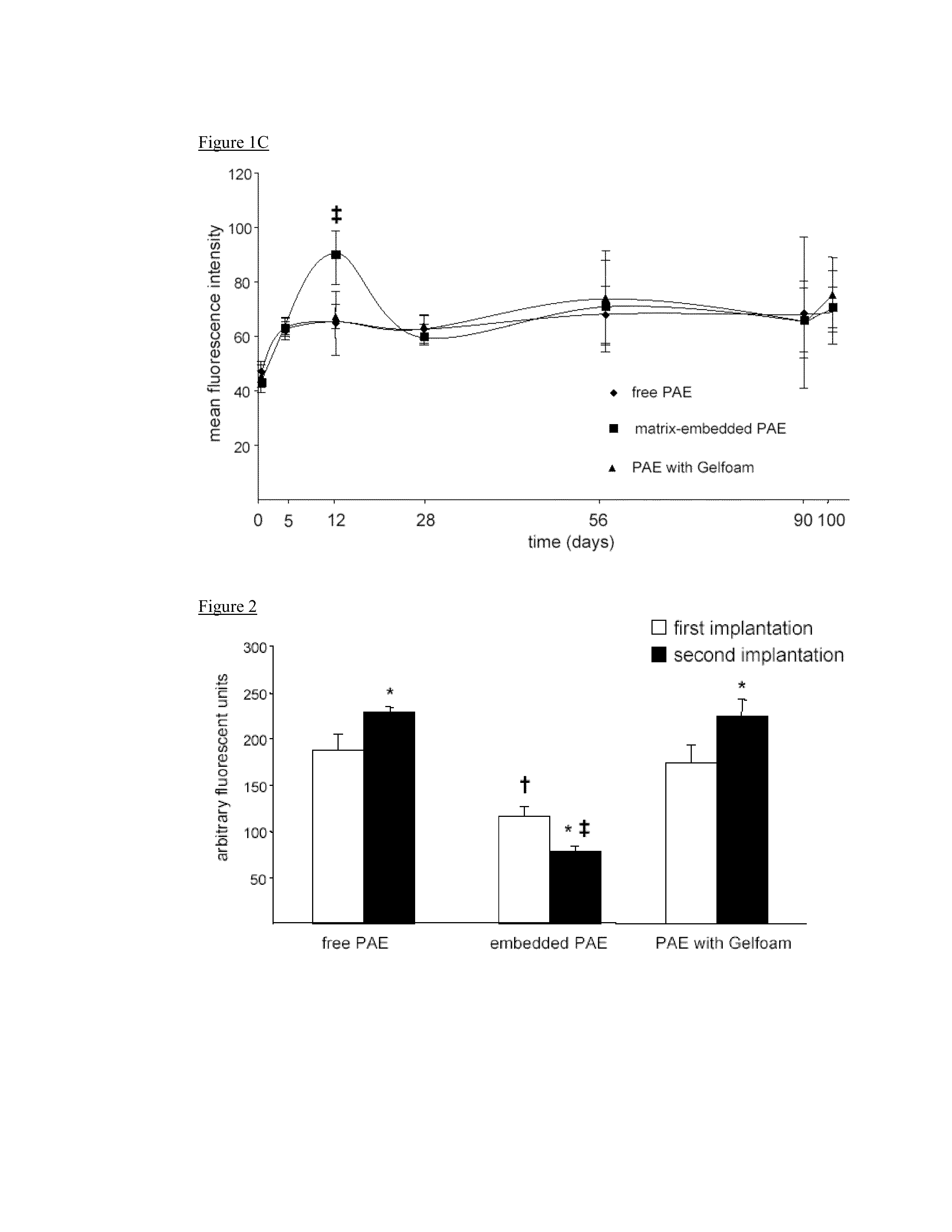
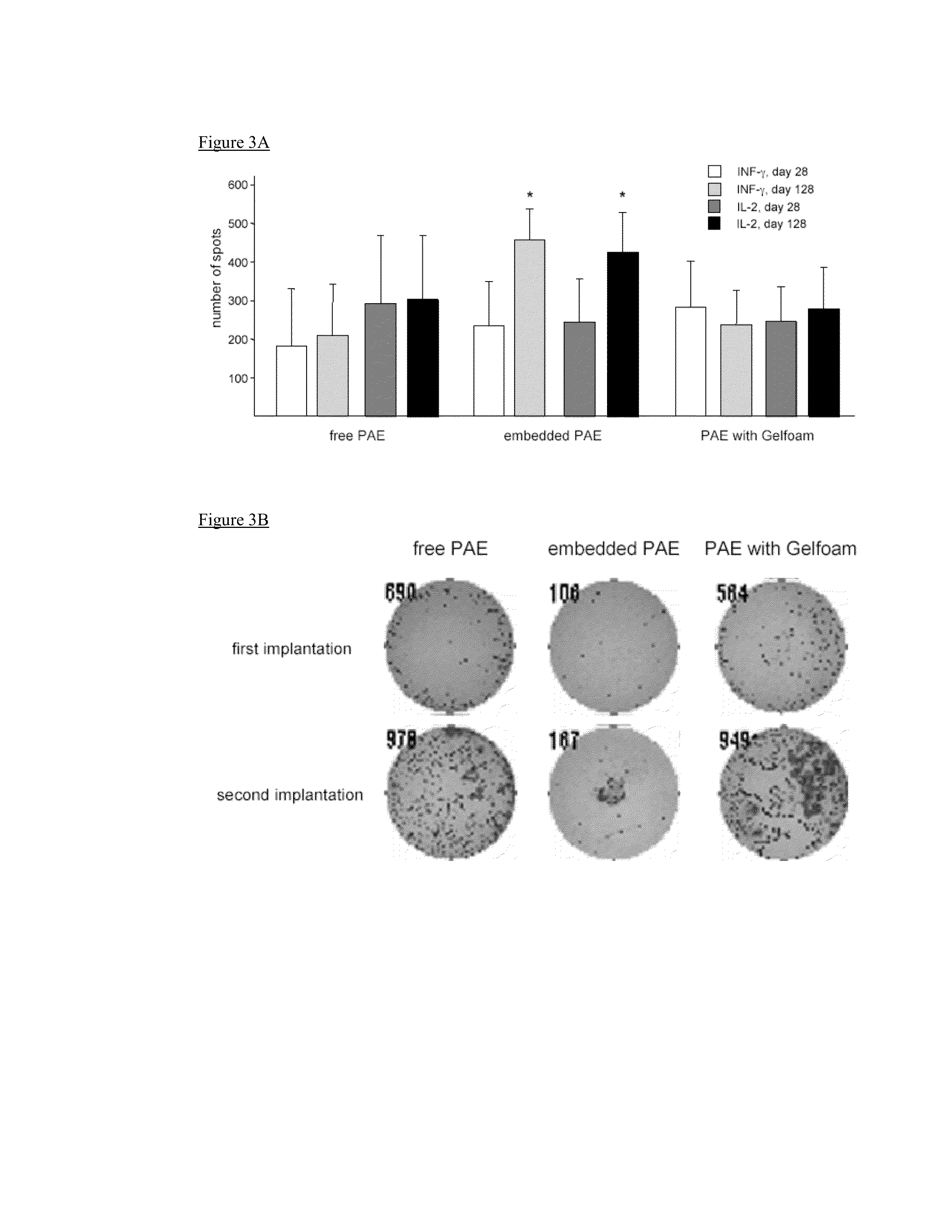
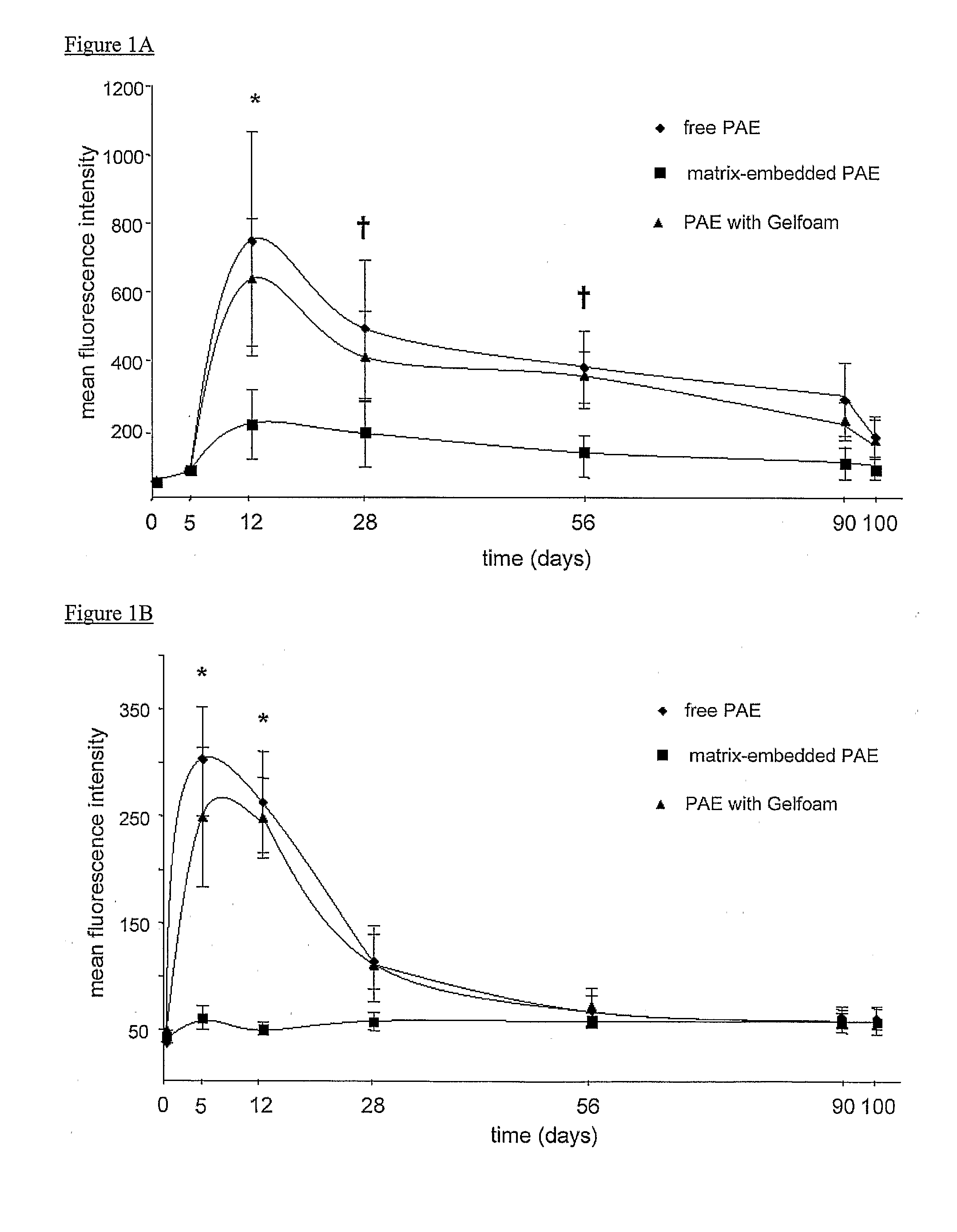
Disclosed herein are materials and methods for modulating an immunologically adverse response to an exogenous or endogenous immunogen, including a cell, tissue, or organ associated immunogen. An implantable material comprising cells, such as but not limited to endothelial cells, anchored or embedded in a biocompatible matrix can modulate an adverse immune or inflammatory reaction to exogenous or endogenous immunogens, including response to non-syngeneic or syngeneic cells, tissues or organs, exogenous immunogens or stimuli, as well as ameliorate an autoimmune condition. The implantable material can be provided prior to, coincident with, or subsequent to occurrence of the immune response or inflammatory reaction. The implantable material can induce immunological acceptance in a transplant patient, reduce graft rejection and reduce donor antigen immunogenicity.
Owner:EDELMAN ELAZER R +2
Materials and Methods for Altering an Immune Response to Exogenous and Endogenous Immunogens, Including Syngeneic and Non-Syngeneic Cells, Tissues or Organs
InactiveUS20130177600A1Reduce reduction reactionReduced responseAntipyreticAnalgesicsAutoimmune conditionAutoimmune responses
Disclosed herein are materials and methods for modulating an immunologically adverse response to an exogenous or endogenous immunogen, including a cell, tissue, or organ associated immunogen. An implantable material comprising cells, such as but not limited to endothelial cells, anchored or embedded in a biocompatible matrix can modulate an adverse immune or inflammatory reaction to exogenous or endogenous immunogens, including response to non-syngeneic or syngeneic cells, tissues or organs, exogenous immunogens or stimuli, as well as ameliorate an autoimmune condition. The implantable material can be provided prior to, coincident with, or subsequent to occurrence of the immune response or inflammatory reaction. The implantable material can induce immunological acceptance in a transplant patient, reduce graft rejection and reduce donor antigen immunogenicity.
Owner:MASSACHUSETTS INST OF TECH
Materials and methods for altering an immune response to exogenous and endogenous immunogens, including syngeneic and non-syngeneic cells, tissues or organs
InactiveUS20090087414A1Lower immune responseReduce decreaseBiocideAntipyreticAutoimmune conditionAutoimmune responses
Disclosed herein are materials and methods for modulating an immunologically adverse response to an exogenous or endogenous immunogen, including a cell, tissue, or organ associated immunogen. An implantable material comprising cells, such as but not limited to endothelial cells, anchored or embedded in a biocompatible matrix can modulate an adverse immune or inflammatory reaction to exogenous or endogenous immunogens, including response to non-syngeneic or syngeneic cells, tissues or organs, exogenous immunogens or stimuli, as well as ameliorate an autoimmune condition. The implantable material can be provided prior to, coincident with, or subsequent to occurrence of the immune response or inflammatory reaction. The implantable material can induce immunological acceptance in a transplant patient, reduce graft rejection and reduce donor antigen immunogenicity.
Owner:PERVASIS THERAPEUTICS INC
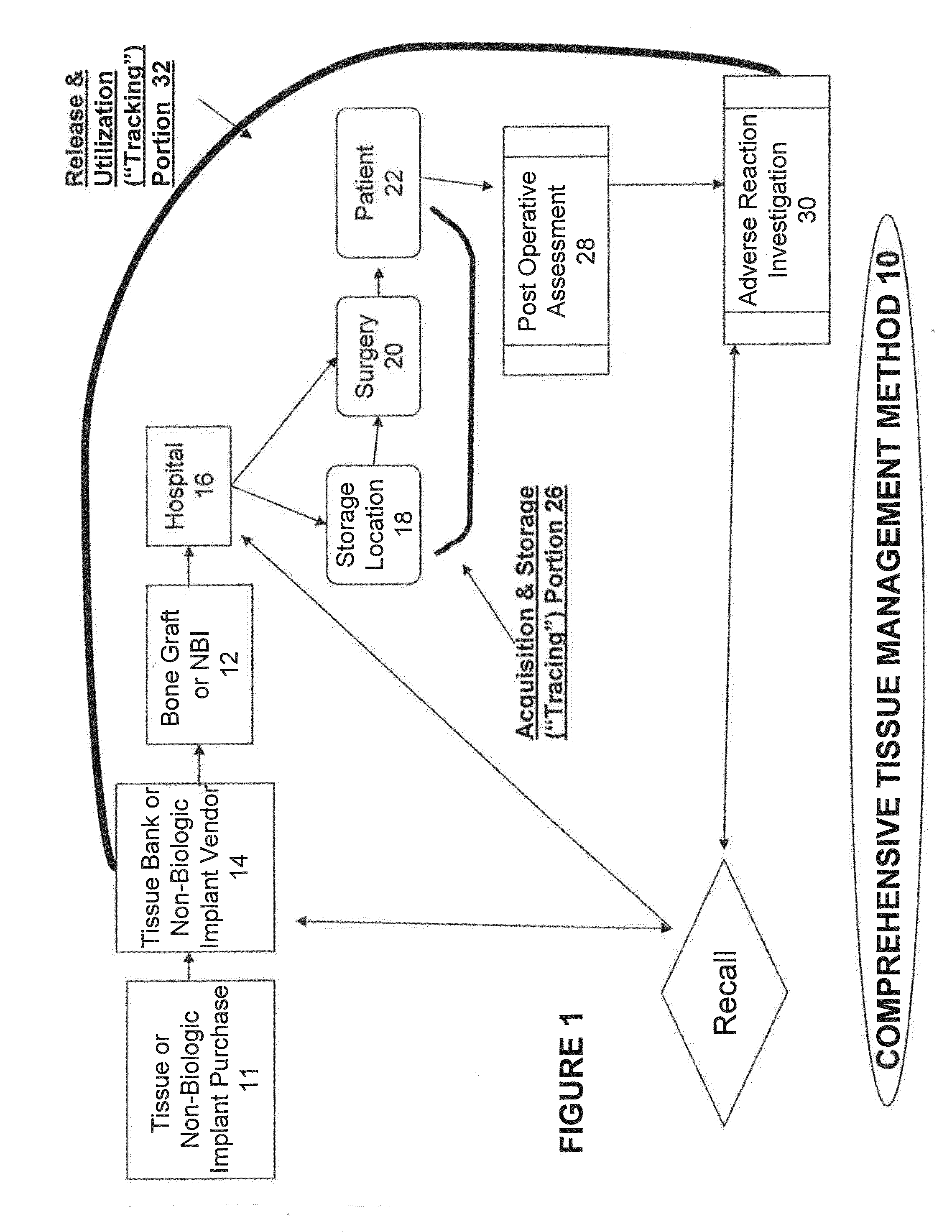
Tissue management system
ActiveUS20080215363A1Mechanical/radiation/invasive therapiesComputer-assisted treatment prescription/deliveryManagement systemMedical treatment
The present invention provides a comprehensive tissue management system for transplantable materials like tissues and organs. The tracking portion of the system prompts and verifies that staff members of a medical establishment like a hospital have handled, stored, transported, reconstituted, and used the tissue or organ materials in a safe and regulatory-compliant manner from the point of receipt to the point of issuance or surgical use throughout the hospital's organization. The tracing portion of the system creates an integral record that documents which hospital staff members have provided which processing steps to the tissue or organ, any associated materials used in conjunction with such tissue or organ, and an identification of the tissue or organ that was transplanted or implanted inside a patient. Such a system will enable adverse reaction investigations for transplant patients, and recalls of transplantable materials.
Owner:BIOMEDICAL SYNERGIES
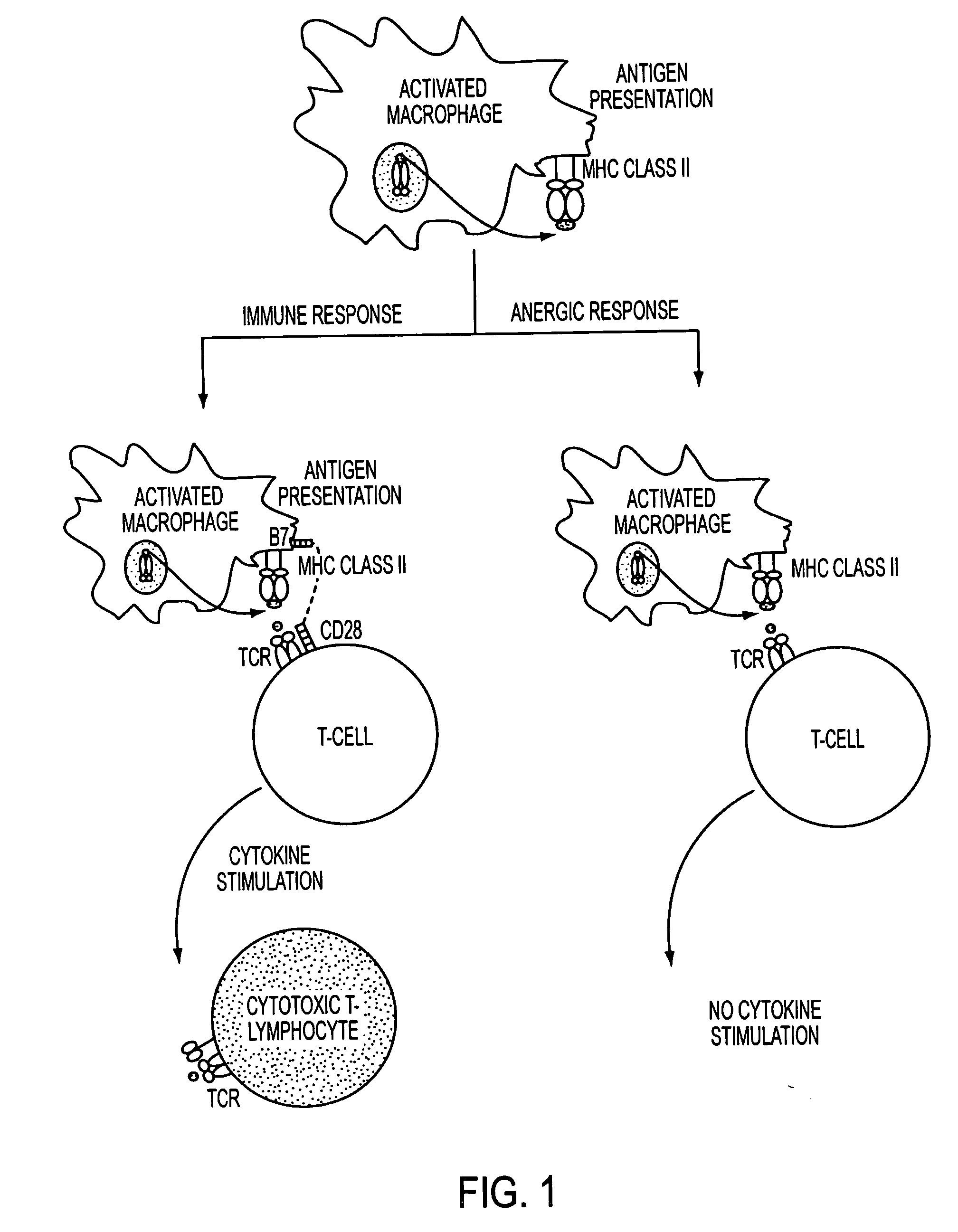
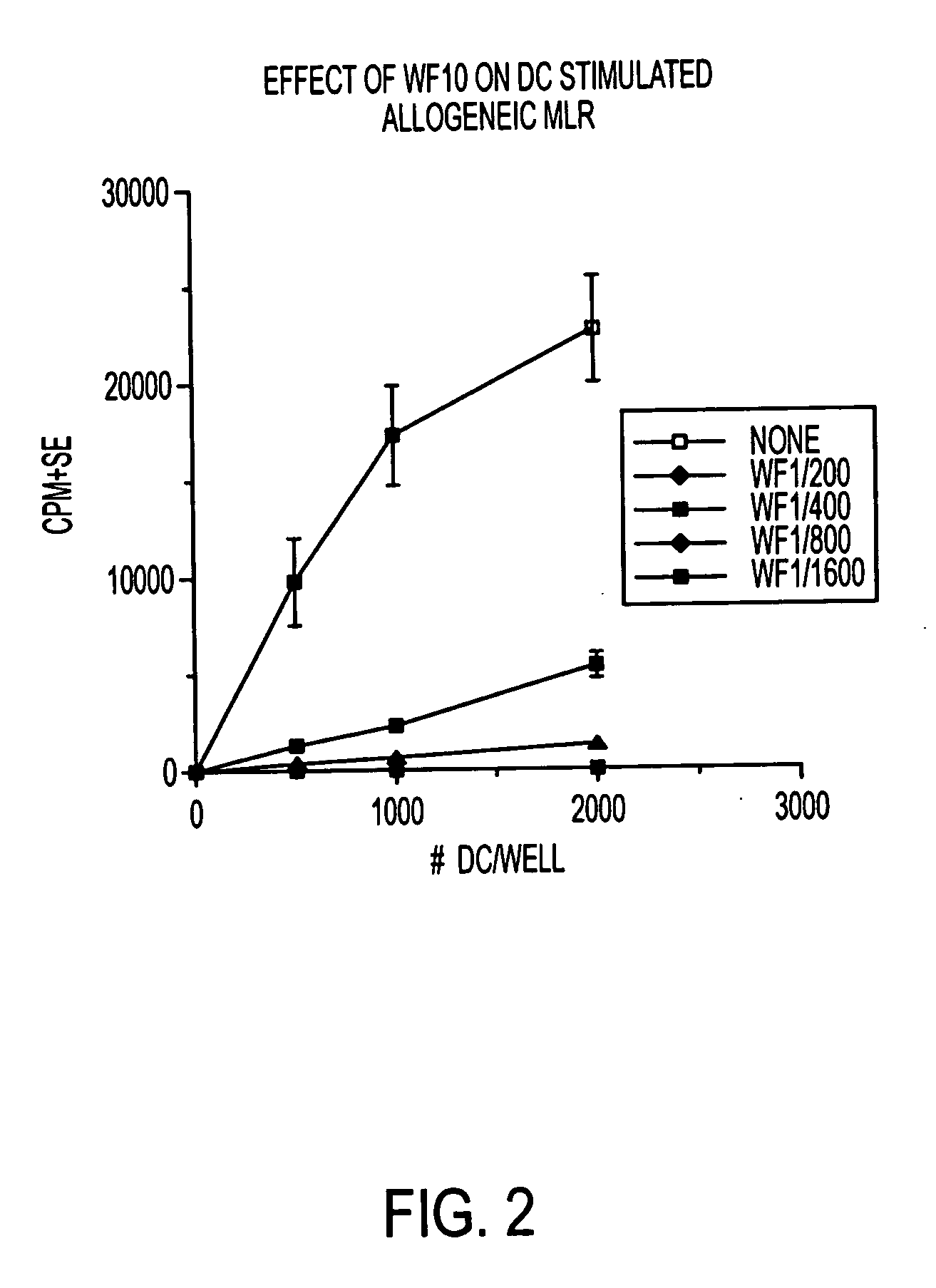
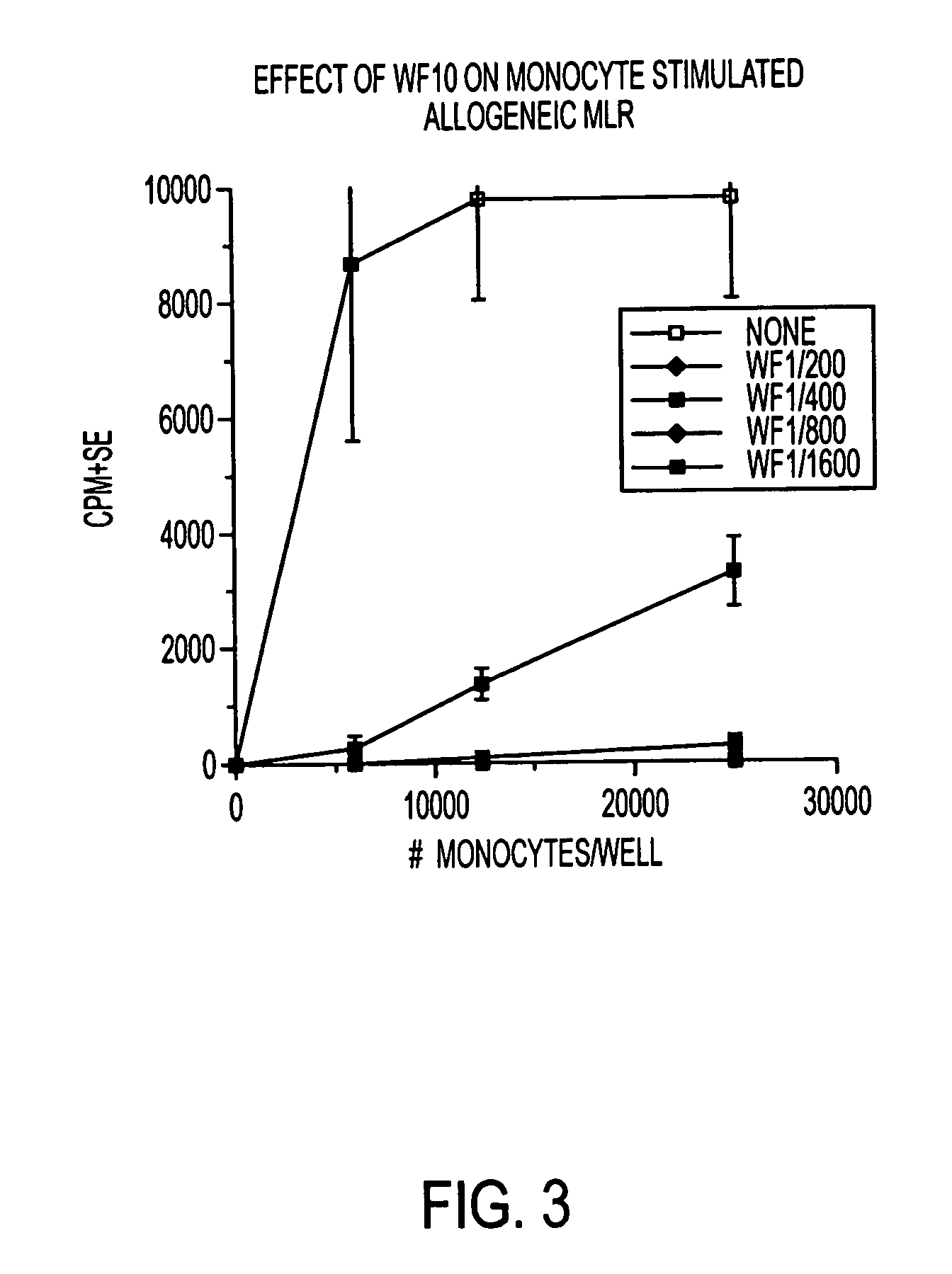
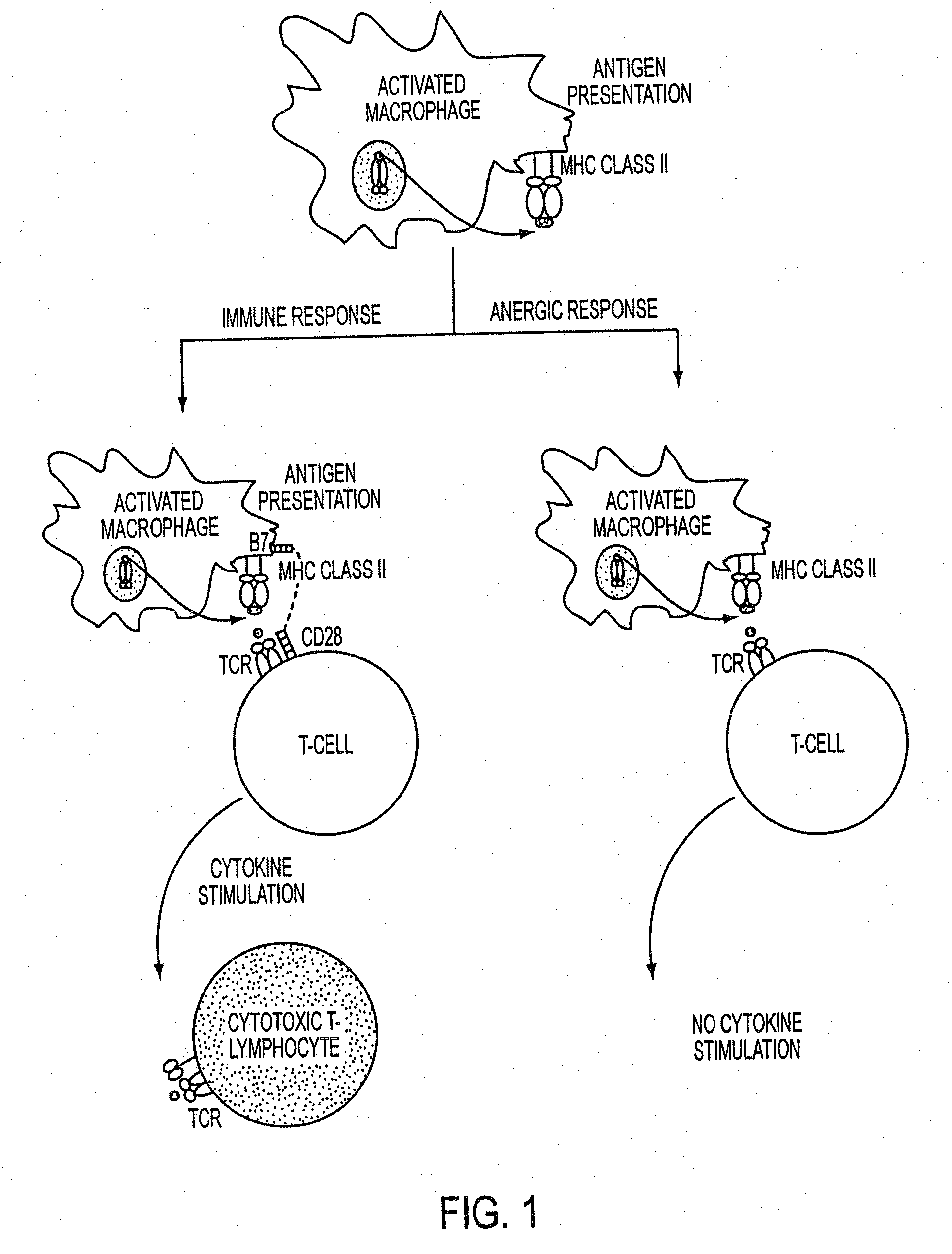
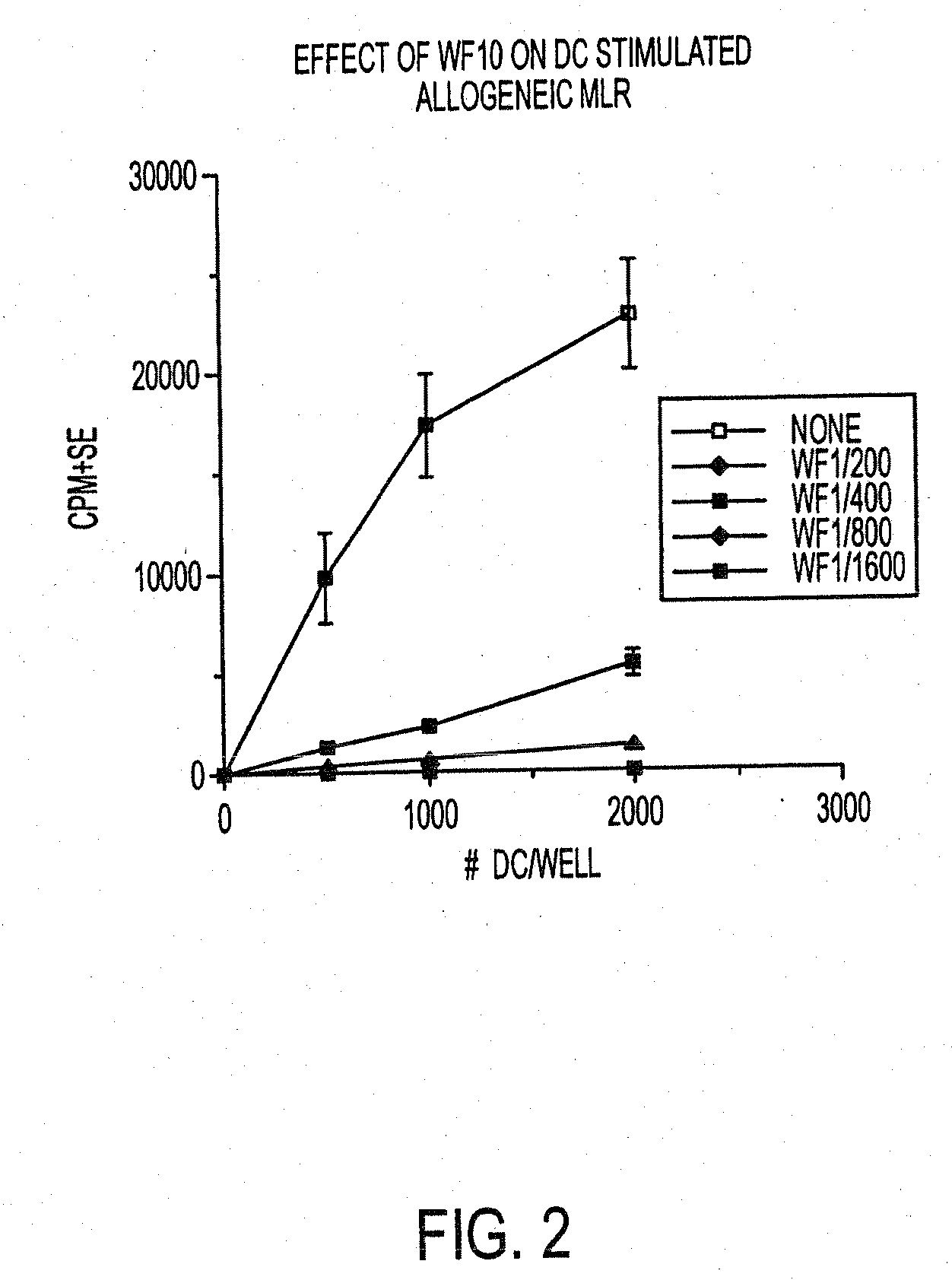
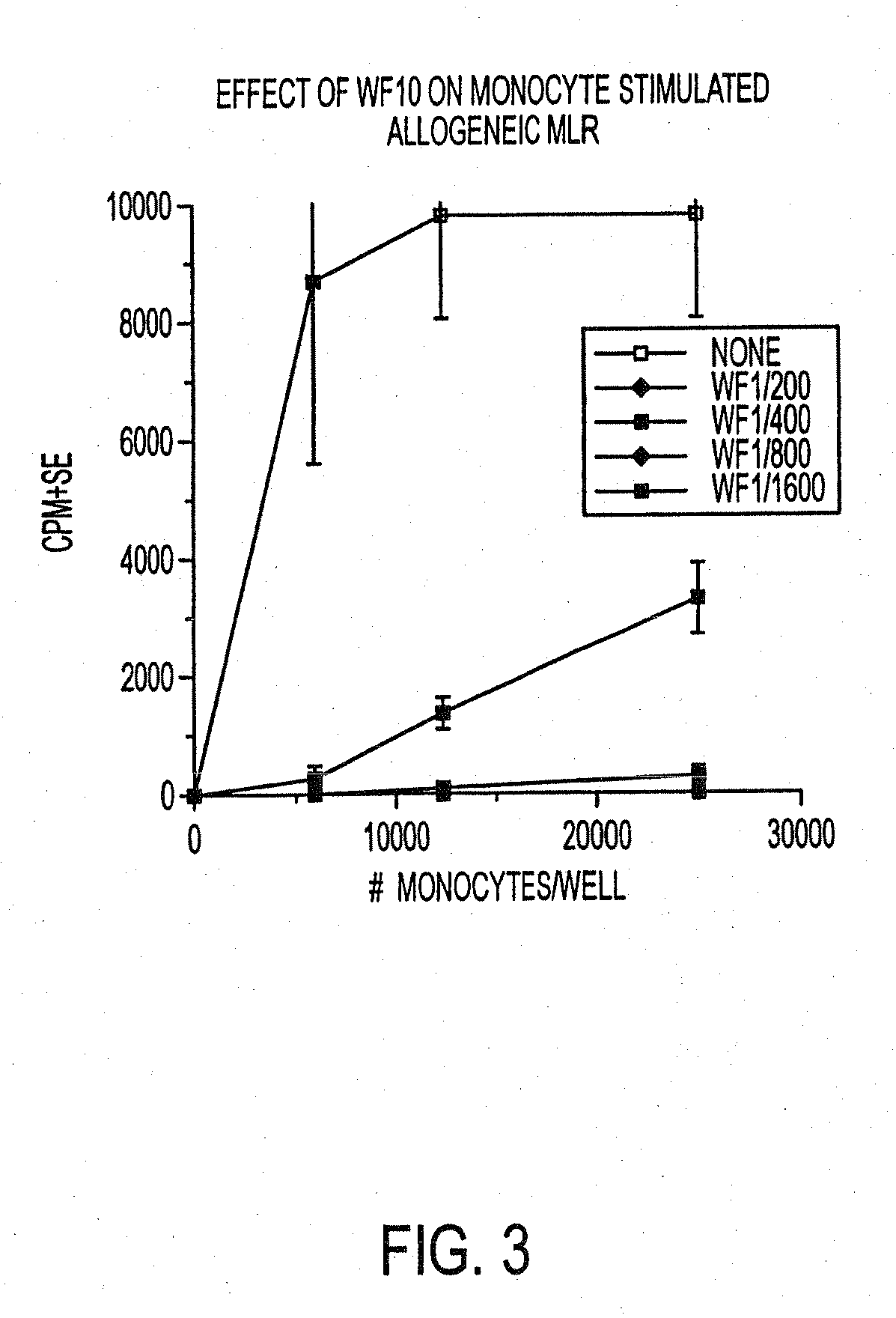
Use of a chemically stabilized chlorite solution for inhibiting an antigen-specific immune response
Methods of using a stabilized chlorite solution to inhibit antigen-specific immune responses are disclosed. The stabilized chlorite solution, when administered to a mammal in need thereof, can prevent the presentation of antigens by antigen presenting cells. The stabilized chlorite solution therefore is useful in treating, inter alia, auto-immune diseases, treating diseases caused by an inappropriate immune response, treating lymphoproliferative disease and in inhibiting rejection in transplant patients.
Owner:NUVO RES
Tissue management system
ActiveUS8666762B2Computer-assisted medical data acquisitionDiagnostic recording/measuringManagement systemMedical treatment
The present invention provides a comprehensive tissue management system for transplantable materials like tissues and organs. The tracking portion of the system prompts and verifies that staff members of a medical establishment like a hospital have handled, stored, transported, reconstituted, and used the tissue or organ materials in a safe and regulatory-compliant manner from the point of receipt to the point of issuance or surgical use throughout the hospital's organization. The tracing portion of the system creates an integral record that documents which hospital staff members have provided which processing steps to the tissue or organ, any associated materials used in conjunction with such tissue or organ, and an identification of the tissue or organ that was transplanted or implanted inside a patient. Such a system will enable adverse reaction investigations for transplant patients, and recalls of transplantable materials.
Owner:BIOMEDICAL SYNERGIES
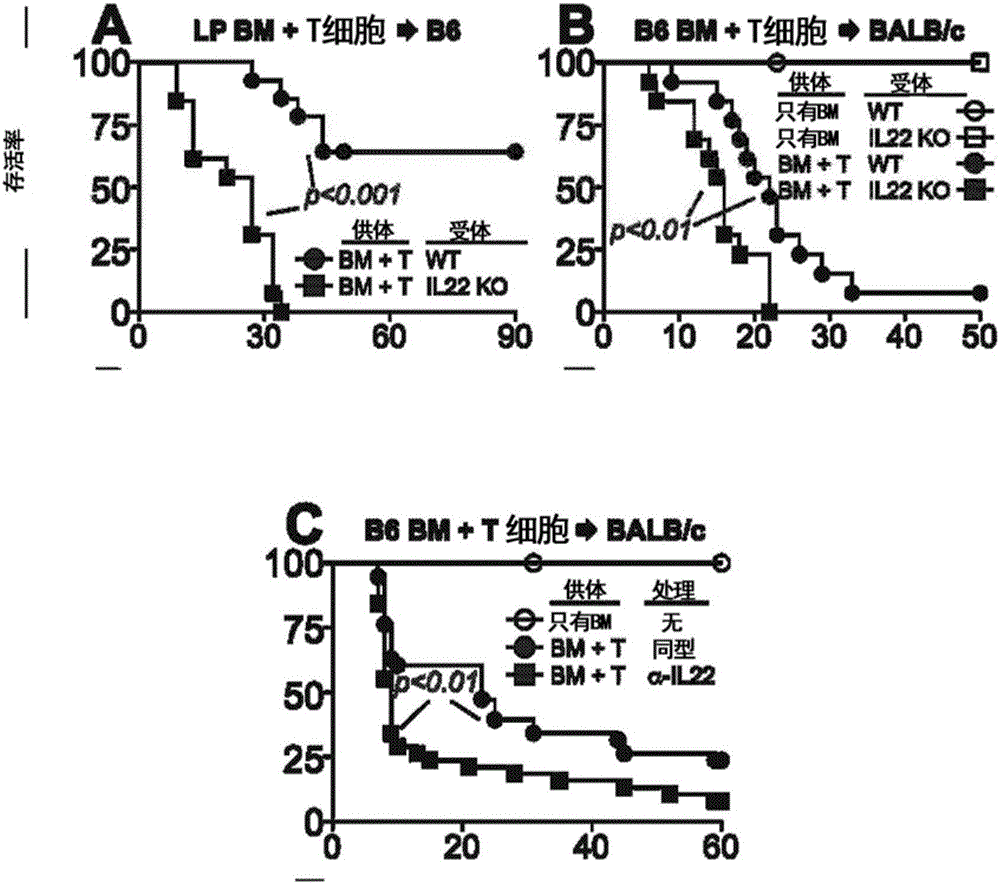
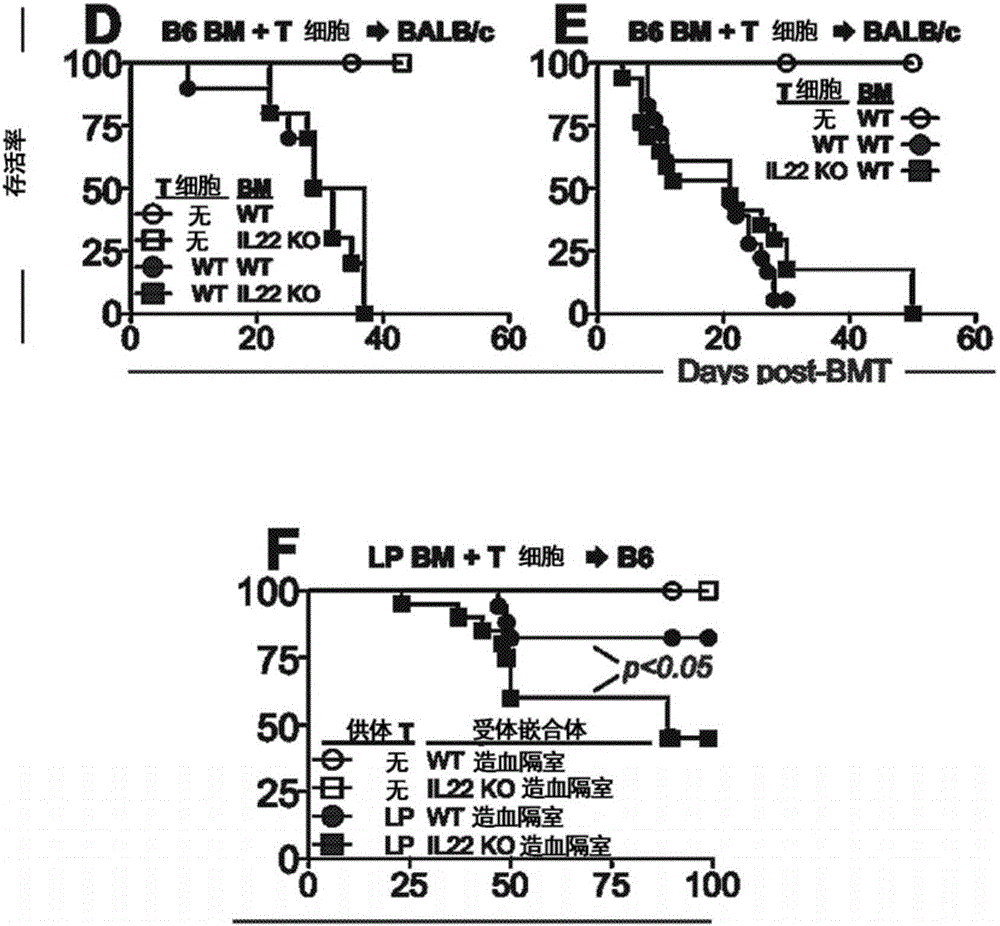
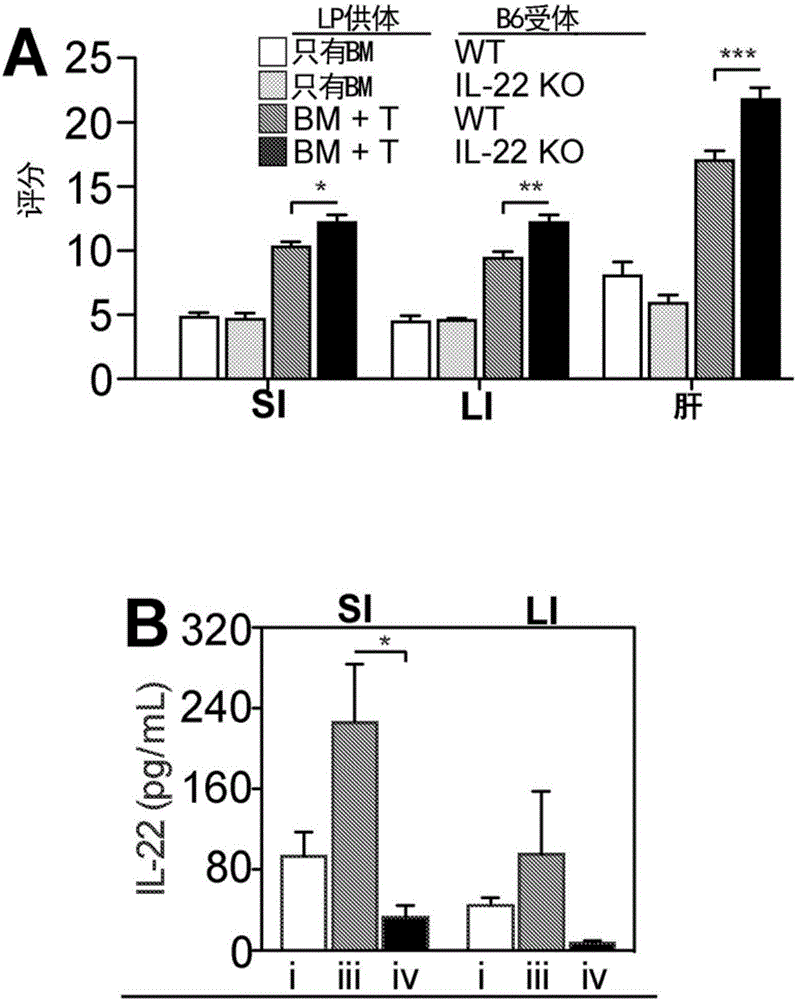
Methods of use for IL-22 in the treatment of gastrointestinal graft vs. host disease
InactiveCN106029100ADisease recoveryPeptide/protein ingredientsDigestive systemHost diseaseGastrointestinal transplantation
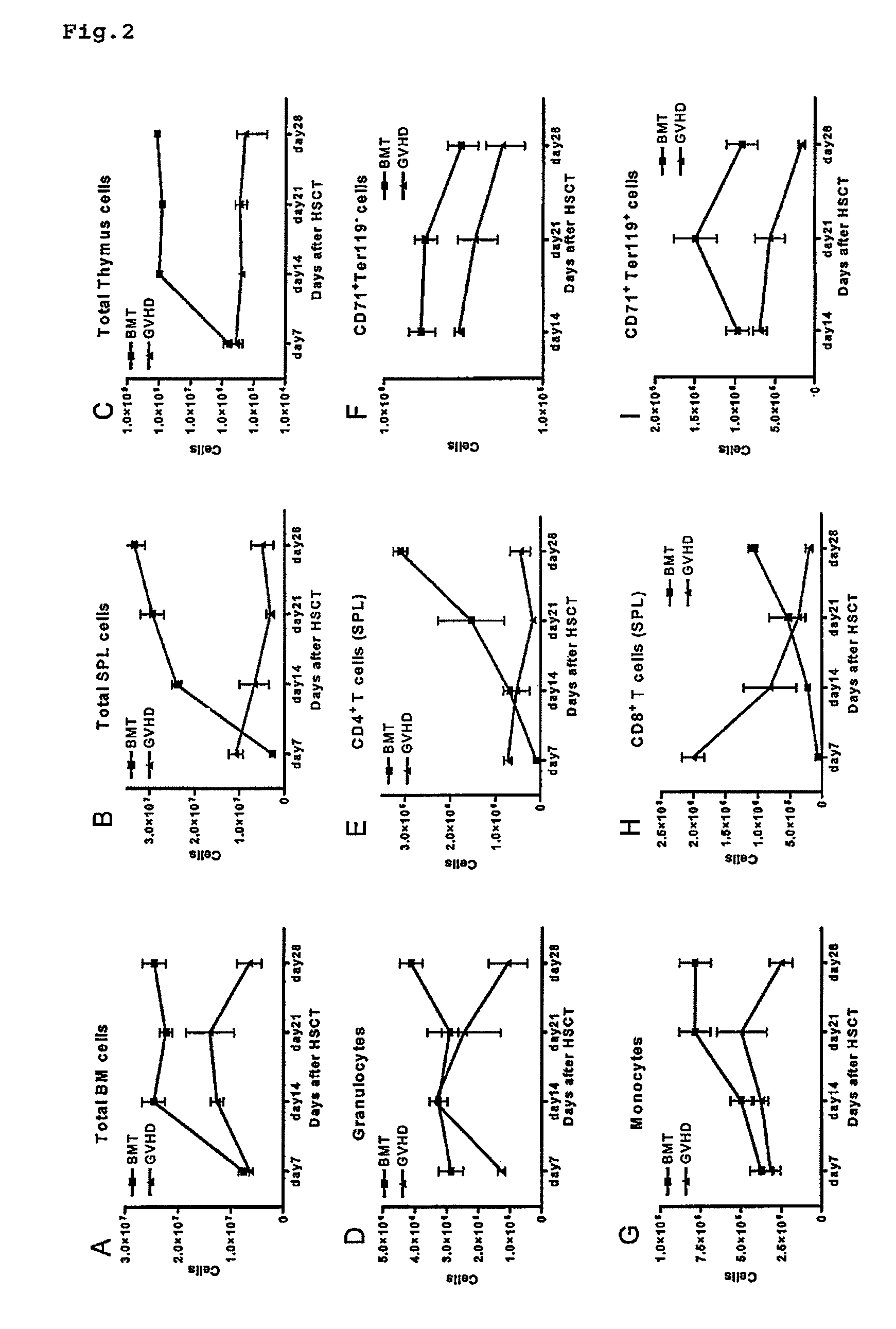
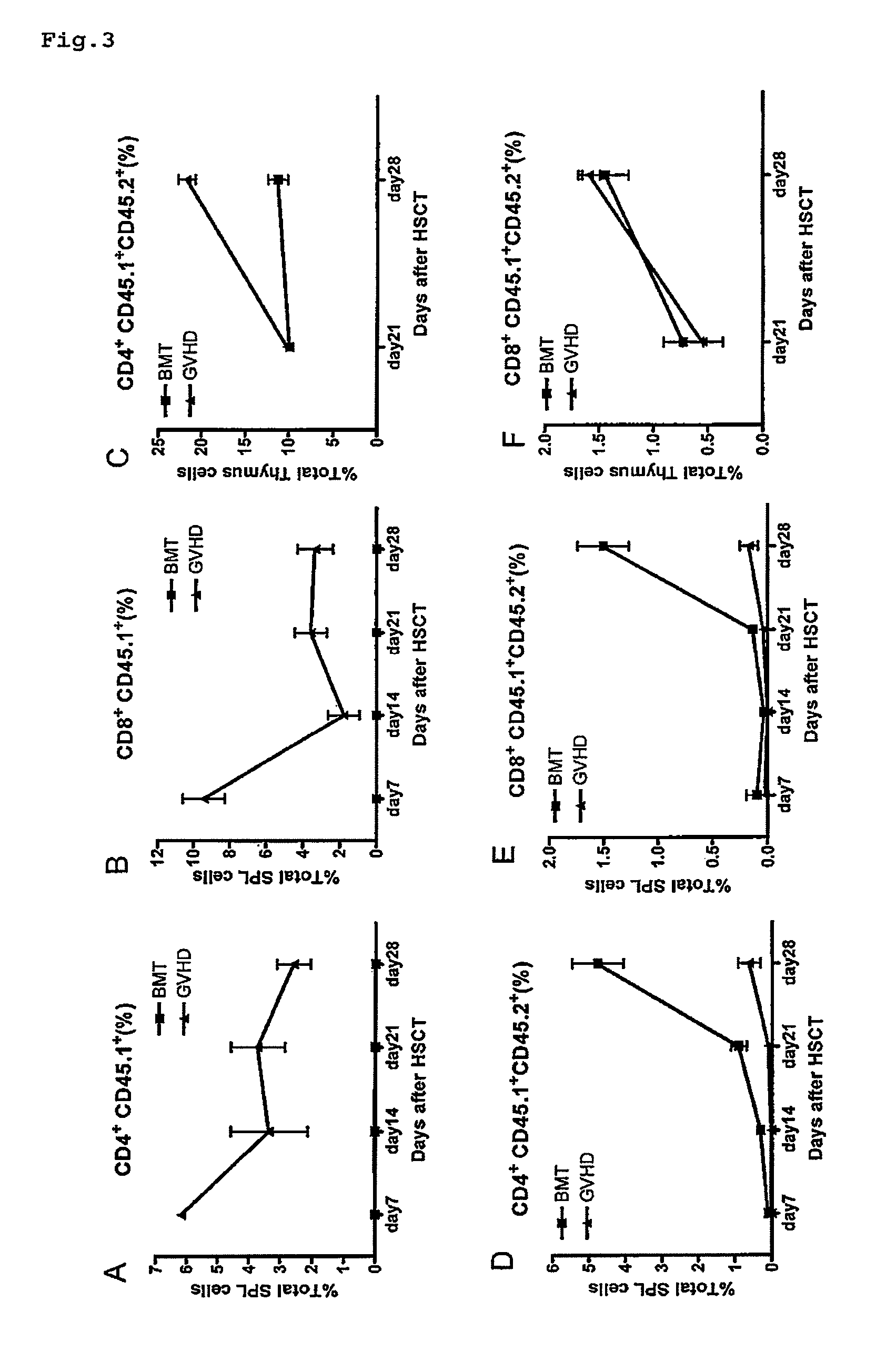
The disclosure provides methods and compositions for the use of IL-22 for treating conditions of intestinal injury and inflammatory conditions such as graft vs. host disease. Specifically, IL-22 can be used to increase Intestinal Stern Cell (ISC) recovery and for enhancing immune reconstitution following allogeneic hematopoietic transplantation. More specifically, the disclosure provides methods of using therapeutic IL-22, including a dimeric form of IL-22, in therapeutic compositions for treating graft vs. host disease, including hepatic, thymic, gastrointestinal, or other graft vs. host disease in hematopoietic stem cell transplant patients and in patients with inflammatory intestinal conditions.
Owner:纪念斯隆–凯特林癌病中心 +1
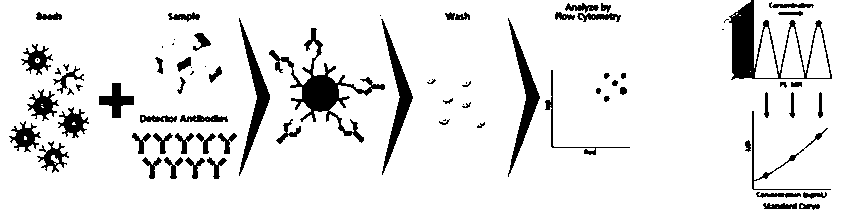
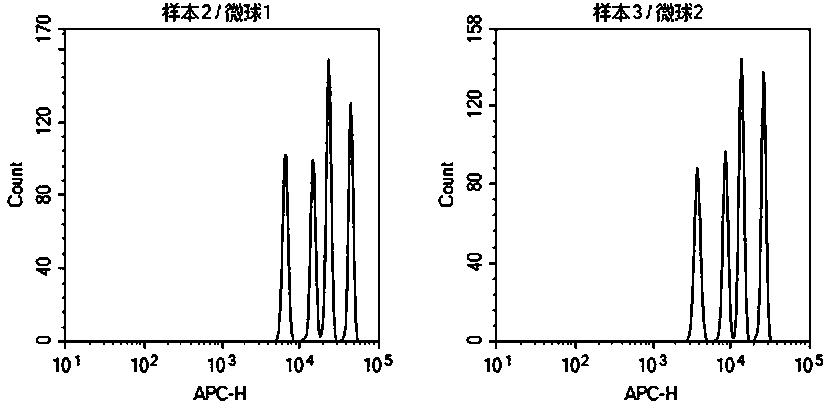
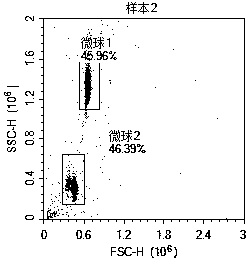
Method for detecting post-transplantation GVHD related cytokines by applying flow cytometry and detection kit
PendingCN110988364AEnable multiple detectionReduce dosageBiological particle analysisDisease diagnosisPost transplantCytokine
The invention provides a method for detecting post-transplantation GVHD related cytokines by using flow cytometry and a detection kit. The method and the detection kit have important clinical significance in the aspects of early evaluation and diagnosis of post-transplantation GVHD. According to the detection method and the kit, indexes of one or more different combinations of cell factors IL-6, IL-8, ST2, Reg3[alpha], Elafin, TNFR1, soluble IL-2R and HGF related to GVHD diagnosis and prognosis can be rapidly and homogeneously detected at the same time; single-sample multiple detection is achieved, time and resources are saved, and the result is automatically analyzed by software so as to be more objective and accurate. The method is simple, convenient, safe in detection and small in sample use quantity. By the application of the method, the risk of the GVHD of the transplanted patient can be evaluated timely, quickly and stably for a long time, the accuracy and timeliness of treatmentof the patient are improved, and the life of the patient is saved.
Owner:天津美瑞特医疗科技有限公司
Tacrolimus For Improved Treatment Of Transplant Patients
InactiveUS20110275659A1Reduce riskAffect bioavailabilityBiocidePharmaceutical non-active ingredientsIn vivoBioavailability
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARMA
Method of Treating Transplant Rejection and Autoimmune Diseases
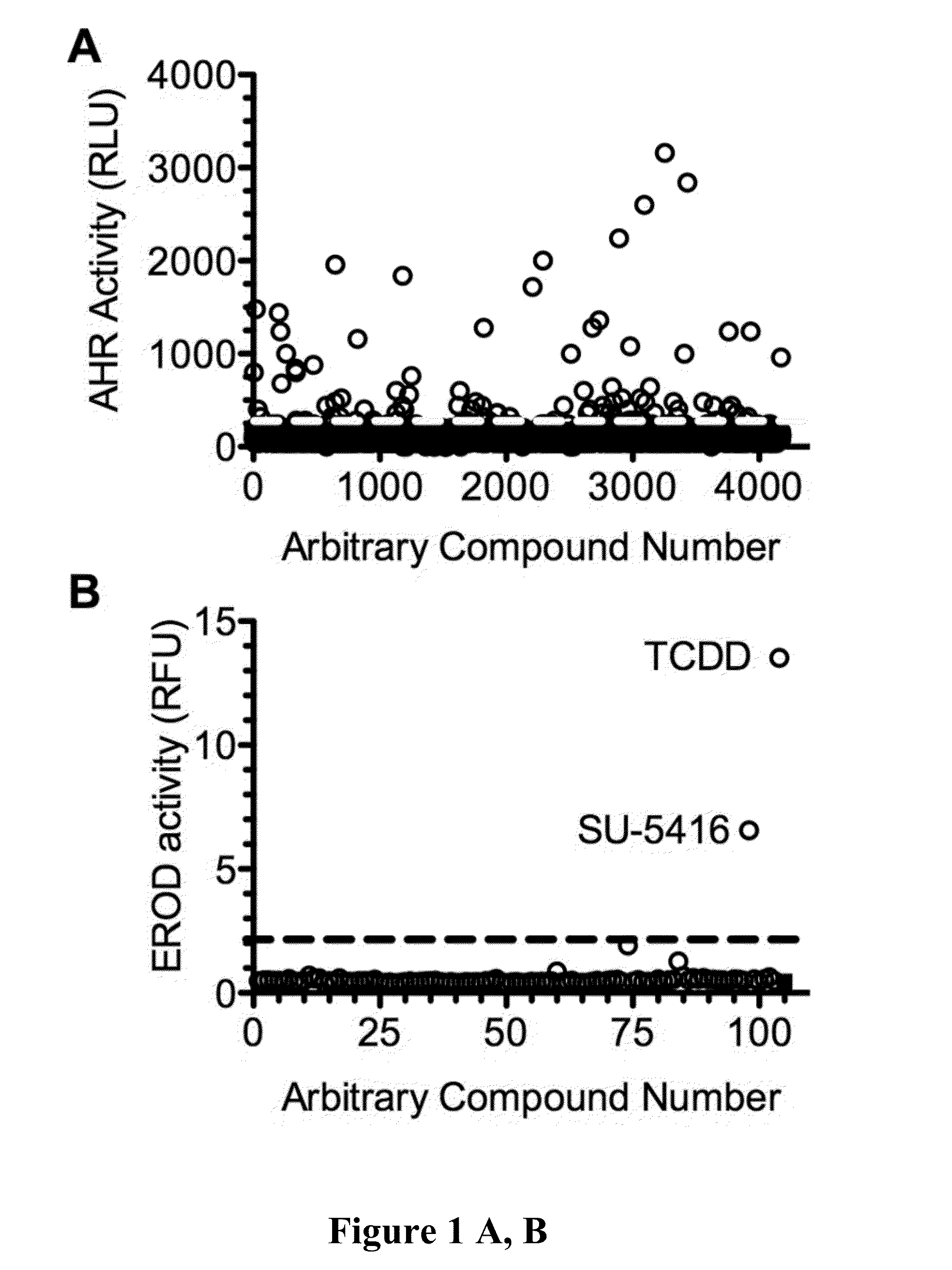
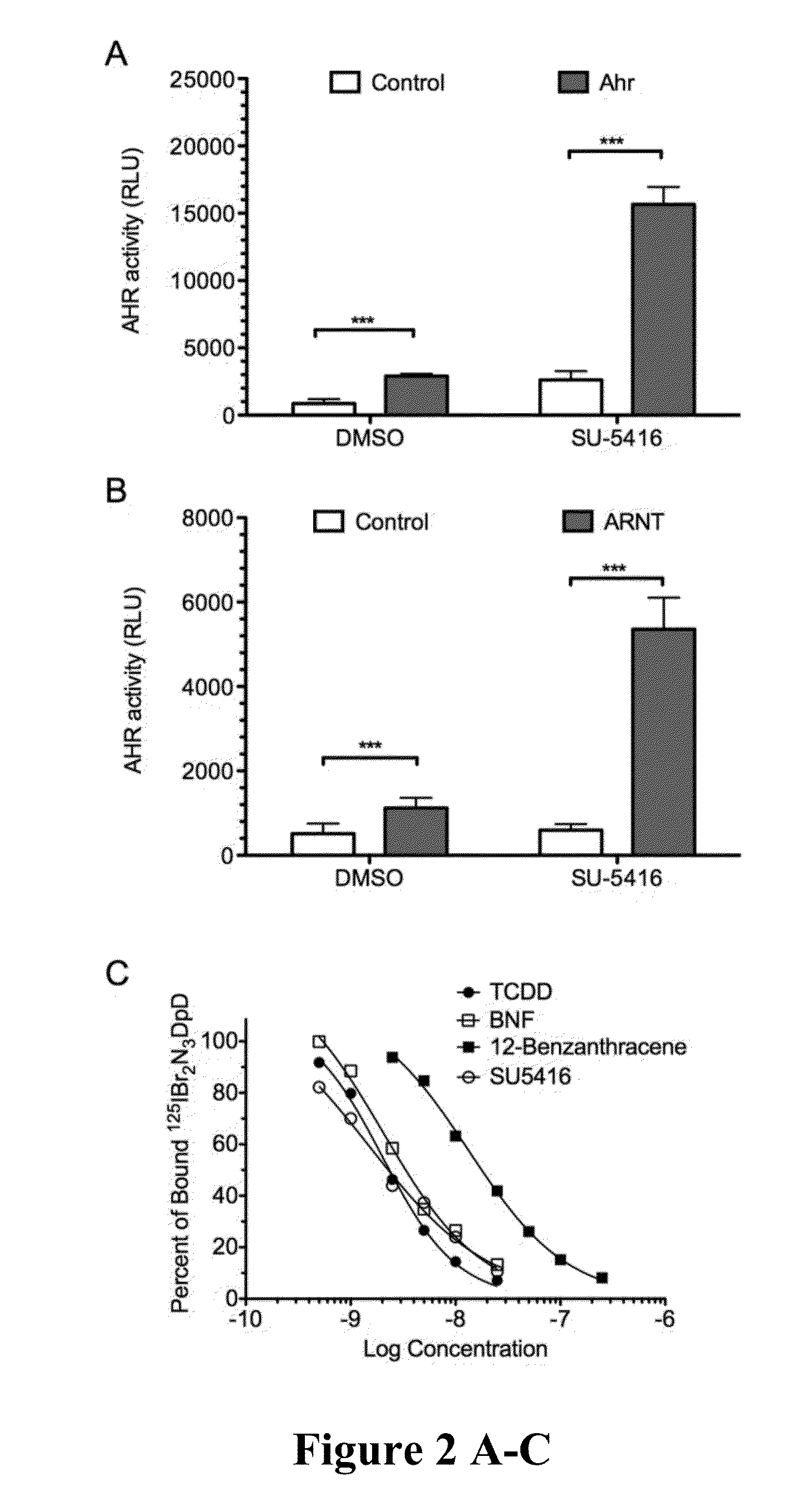
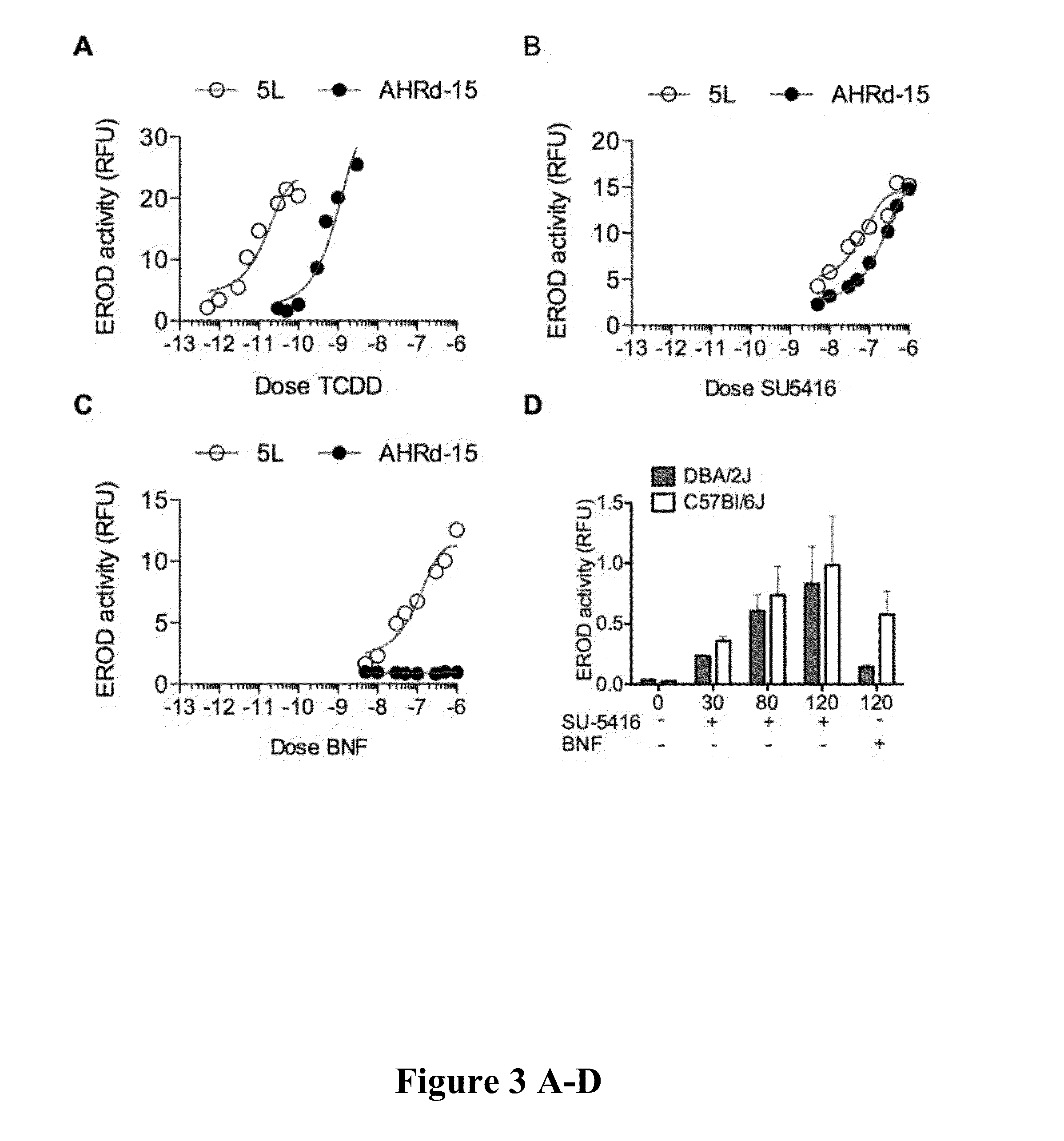
A method of treating autoimmune diseases and transplant rejection, comprising the step of treating the autoimmune or transplant patient with an effective amount of SU-5416 is disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Immunological reconstitution promoter or prophylactic agent for infections each of which maintains graft-versus-tumor effect
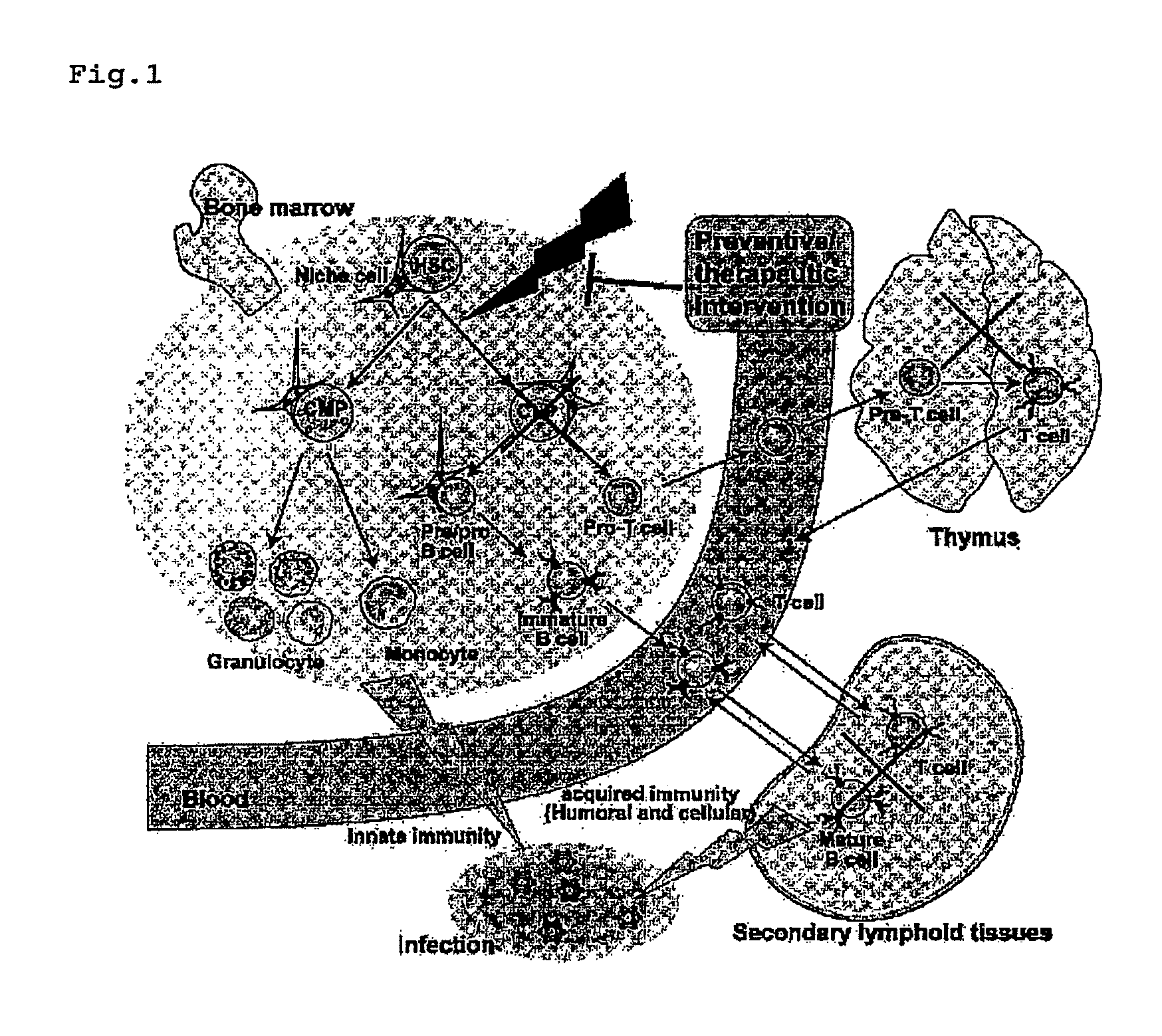
The object of the invention is to provide an immunological reconstitution promoter or a prophylactic agent for infections for use in allogeneic hematopoietic stem cell transplantation therapy for tumors. The promoter or prophylactic agent enables the amelioration of delayed immune reconstitution or the prevention of infection following transplantation, while maintaining the GVT effect of allogeneic hematopoietic stem cell transplantation. Specifically, in a transplant patient in whom immune reconstitution is delayed, such reconstitution can be promoted by administering, at an early stage following transplantation, a substance capable of depleting CD4+ cells. Early completion of infection management in the patient and improvement in the survival rate are anticipated as a result. In addition, the risk of complications associated with allogeneic hematopoietic stem cell transplantation is reduced, enabling more widespread use of this therapy.
Owner:KOUJI MATSUSHIMA
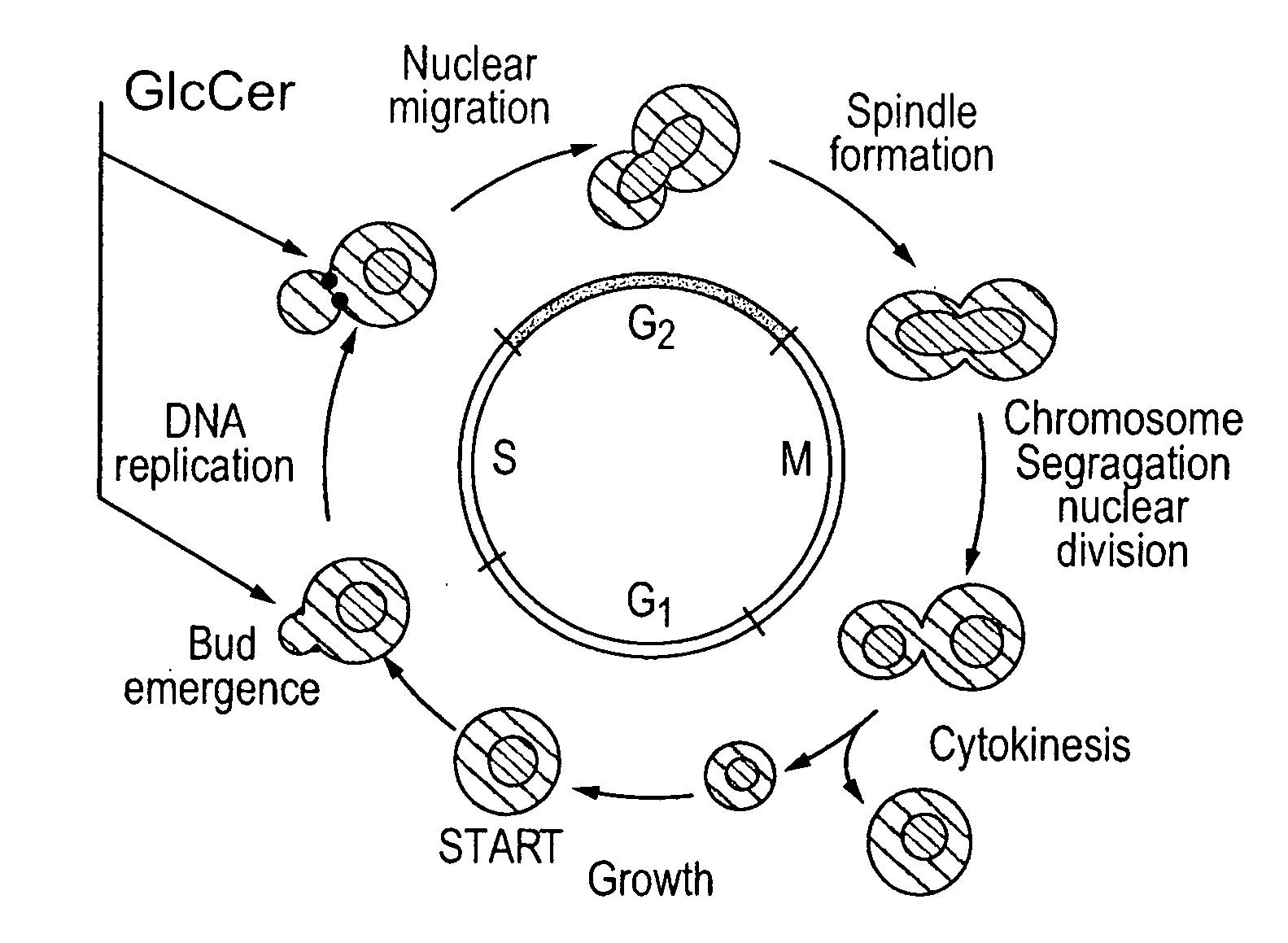
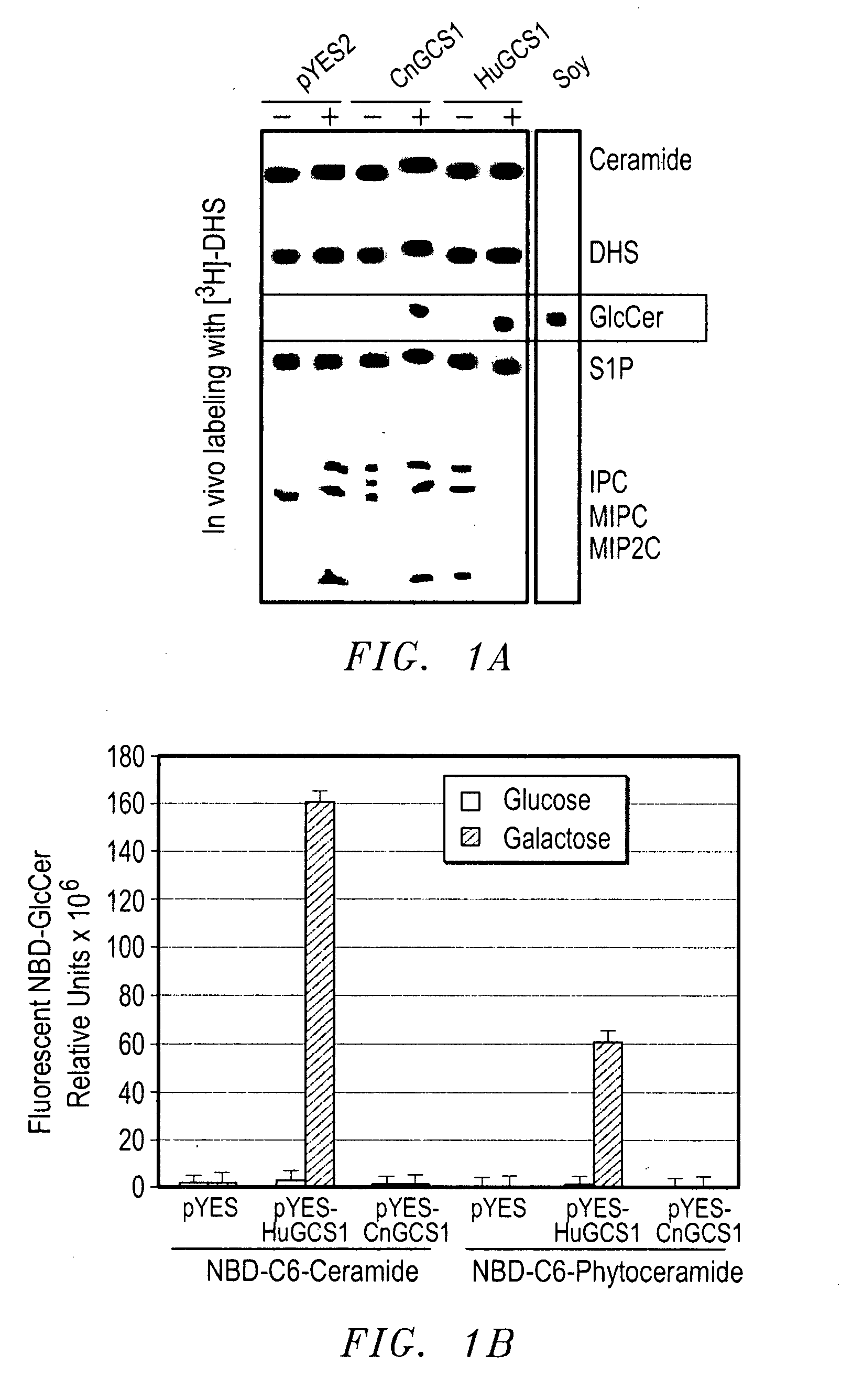
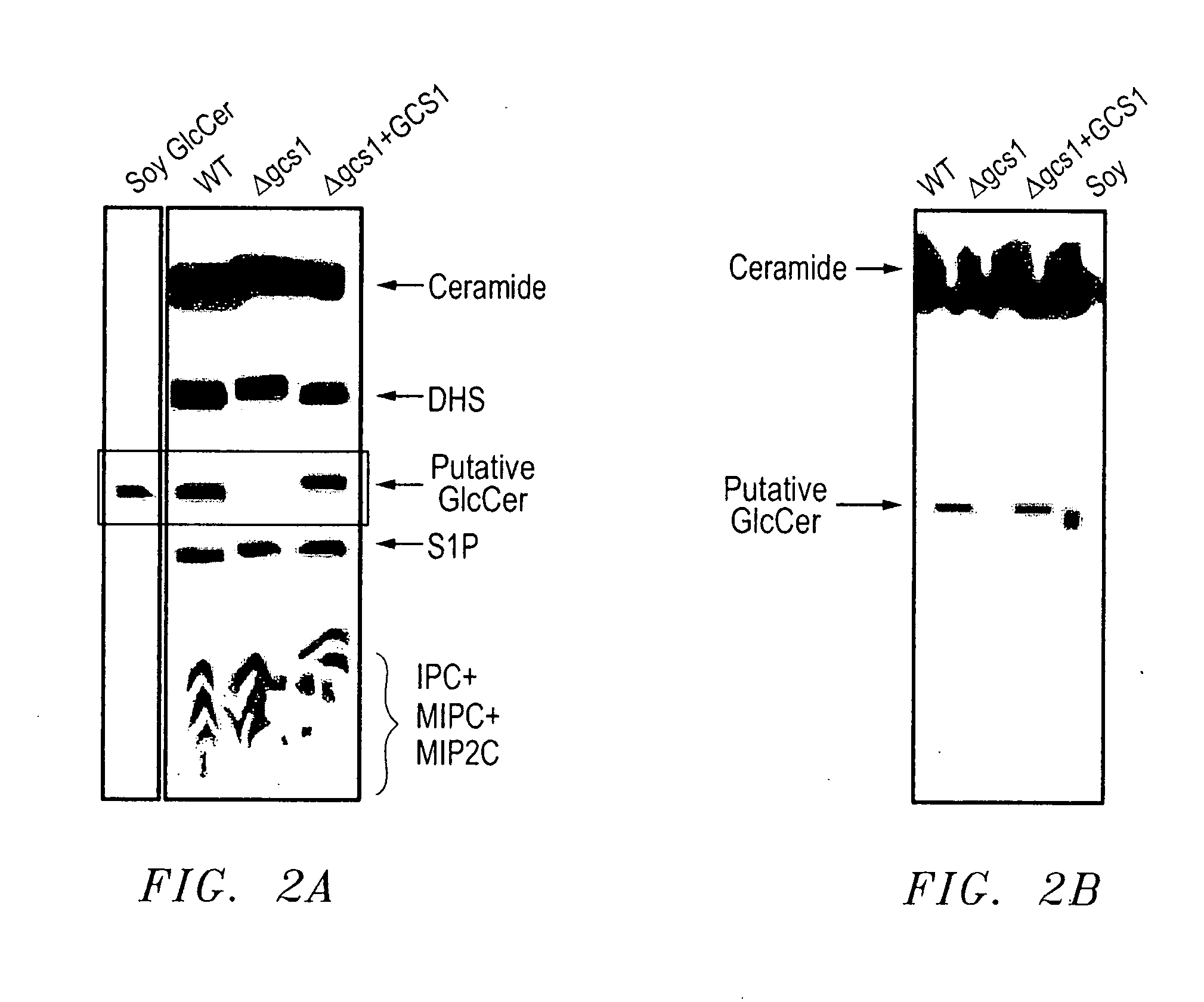
Methods For The Diagnosis And Treatment of Fungal Infections Caused By Microorganisms Producing Glucosylceramide
InactiveUS20080014192A1Avoid spreadingReduce transmissionAntibody ingredientsDrug compositionsImmunosuppressive drugMedical intensive care unit
Methods for the diagnosis and treatment of fungal infections caused by microorganisms producing glucosylceramide are provided. In particular, methods for diagnosing or predicting dissemination of a fungal infection from the lungs to the blood stream of a patient are provided. Methods for treating a fungal infection and / or preventing the dissemination of a fungal infection from the lungs to the blood stream or to other organs of a patient, and methods for preventing or treating a fungal infection in a patient by administering a therapeutically effective amount of a fungal glucosylceramide synthase inhibitor to a patient. Such methods are particularly useful for use with patients at risk to develop fungal infections, such as HIV or cancer patients, patients hospitalized in intensive care units, or patients receiving immunosuppressive drugs such as transplant patients.
Owner:MUSC FOUND FOR RES DEV
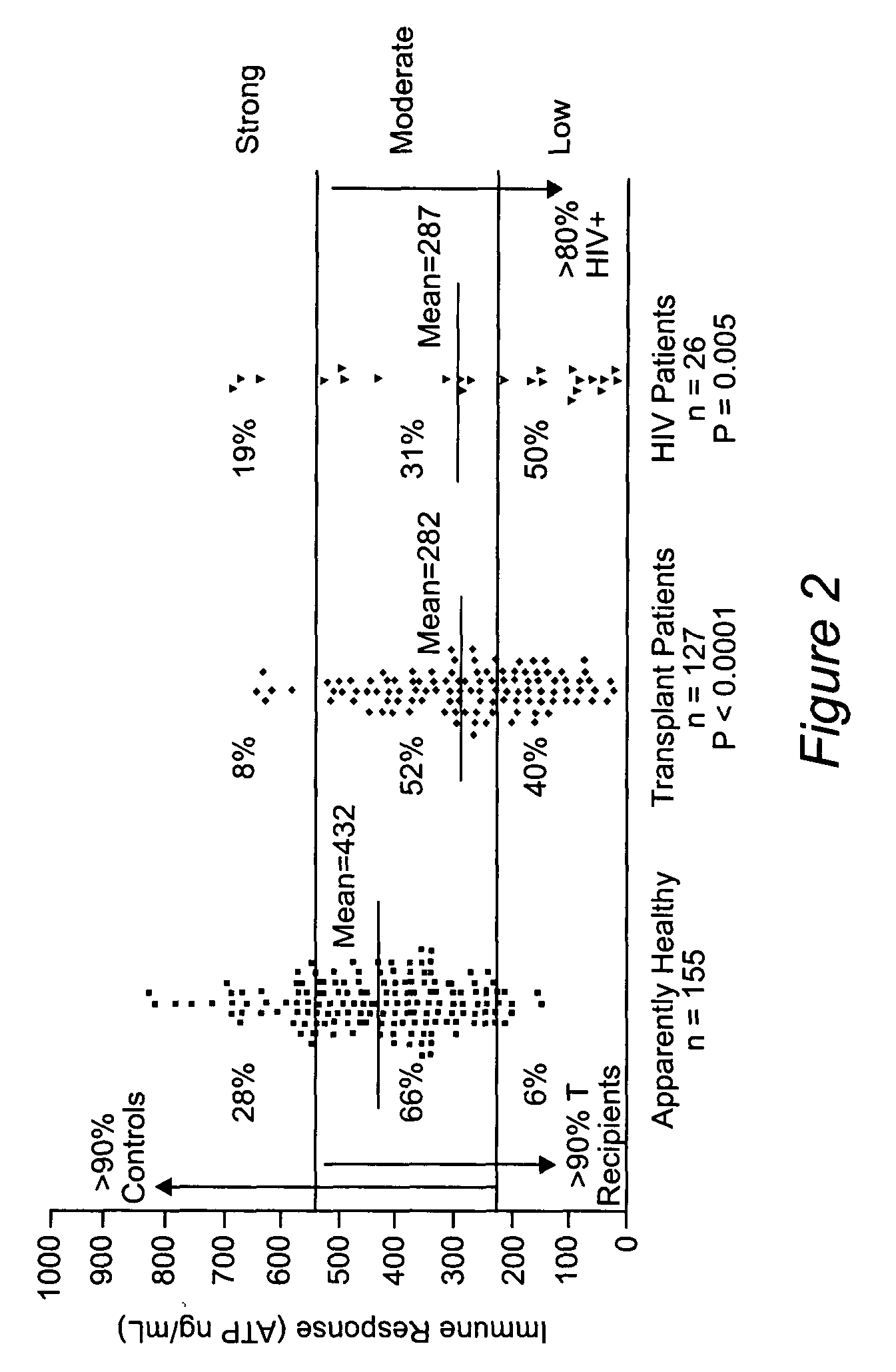
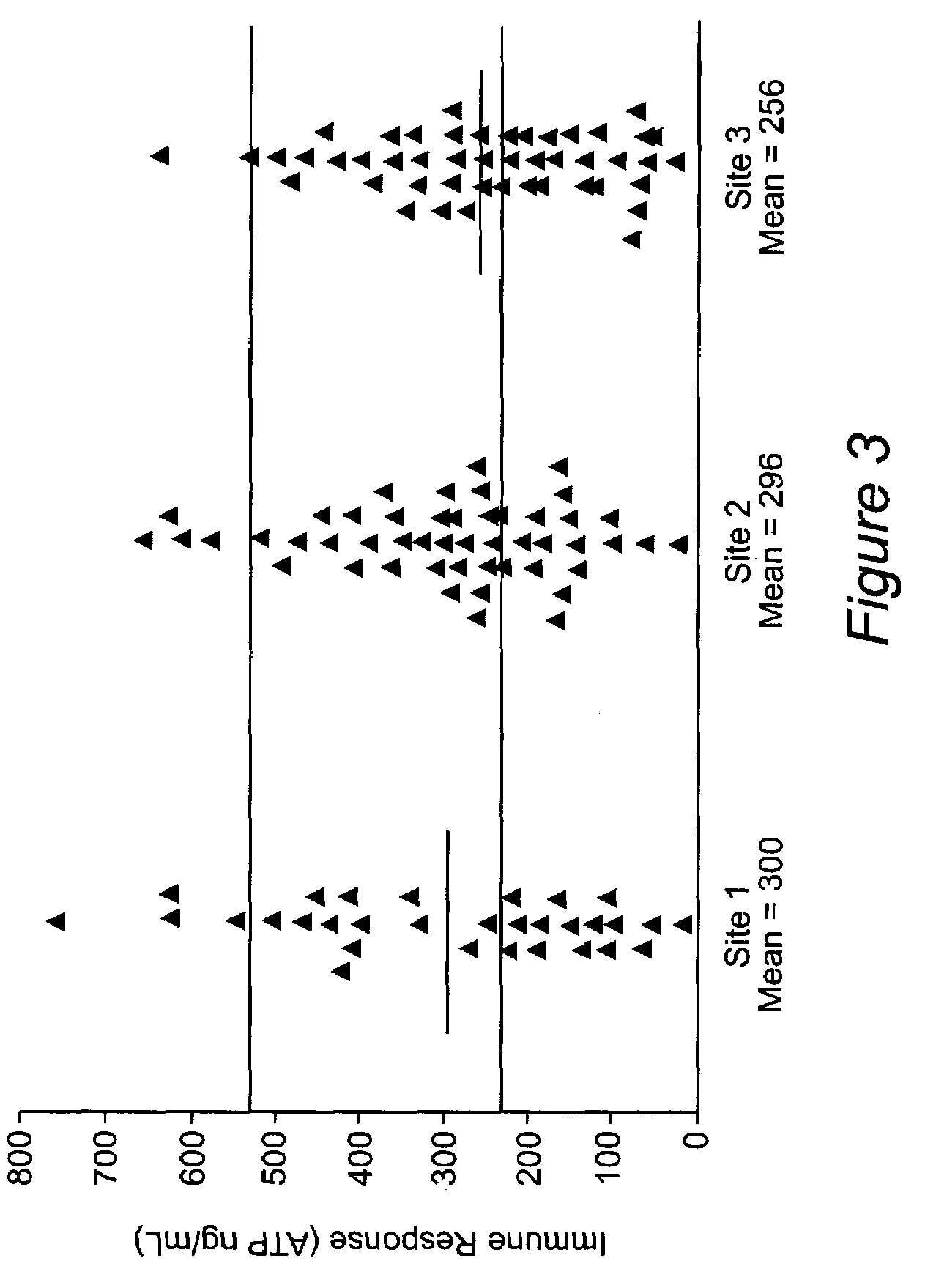
Method for monitoring the immune response and predicting clinical outcomes in transplant recipients
ActiveUS7476514B2Microbiological testing/measurementDisease diagnosisImmunosuppressive drugLymphocyte
Methods for monitoring the immune response and predicting clinical outcomes for patients on immunosuppressive drugs (such as transplant patients) are provided. The methods are based on the measurement of an intracellular metabolic marker in lymphocytes (such as ATP) as an indicator of a patient's immune response.
Owner:EUROFINS VIRACOR INC
Use of a chemically-stabilized chlorite solution for inhibiting an antigen-specific immune response
Owner:NUVO RES
Supperssor of the endogenous interferon-gamma
InactiveUS20110135602A1Suppressing hIFN-γ activityNervous disorderPeptide/protein ingredientsDiseaseAutoimmune responses
The invention relates to suppressor of the endogenous human interferon-gamma (hlFN-γ) applicable in treatment of diseases associated with impaired activity of endogenous hlFN-γ, especially autoimmune diseases and for prevention of graft arteriosclerosis and rejection of organs in allograft transplanted patients. It is based on inactive analogues of the hlFN-γ with pre-served affinity to the hlFN-γ receptor, genetically modified in the domain responsible for triggering the signal transduction pathway.
Owner:MILLICOM
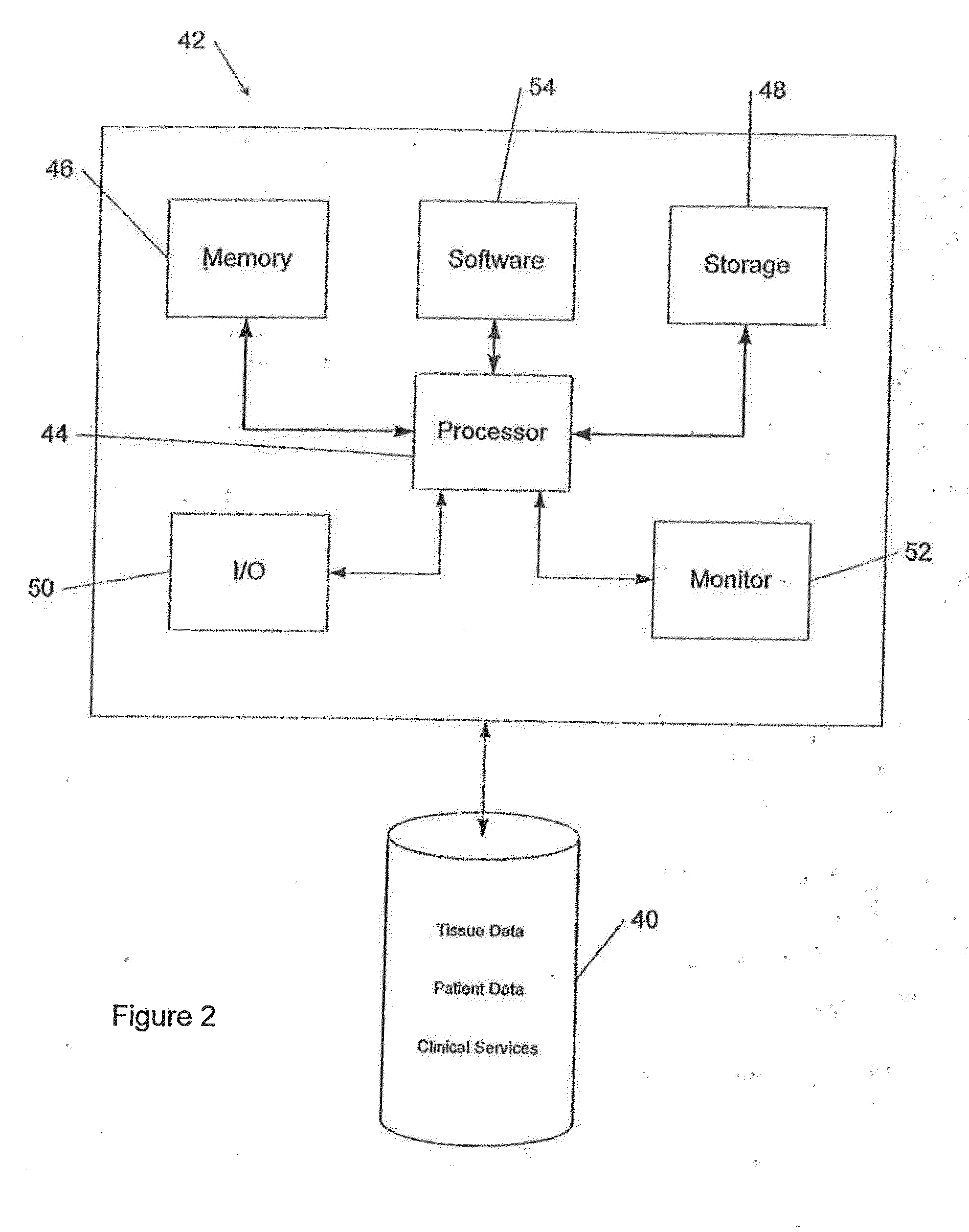
Comprehensive tissue management system
InactiveUS20080077432A1Data processing applicationsDiagnostic recording/measuringMedical institutionSurgery
The present invention provides a comprehensive tissue management method and system for transplantable materials like tissues and organs. The tracking portion of the system verifies that staff members of a medical establishment like a hospital have handled, stored, transported, reconstituted, and used the tissue or organ materials in a safe and regulatory-compliant manner from the point of receipt to the point of issuance or surgical use throughout the hospital's organization. The tracing portion of the system creates an integral record that documents which hospital staff members have provided which processing steps to the tissue or organ, any associated materials used in conjunction with such tissue or organ, and an identification of the tissue or organ that was transplanted or implanted inside a patient. Such a system will enable adverse reaction investigations for transplant patients.
Owner:BIOMEDICAL SYNERGIES
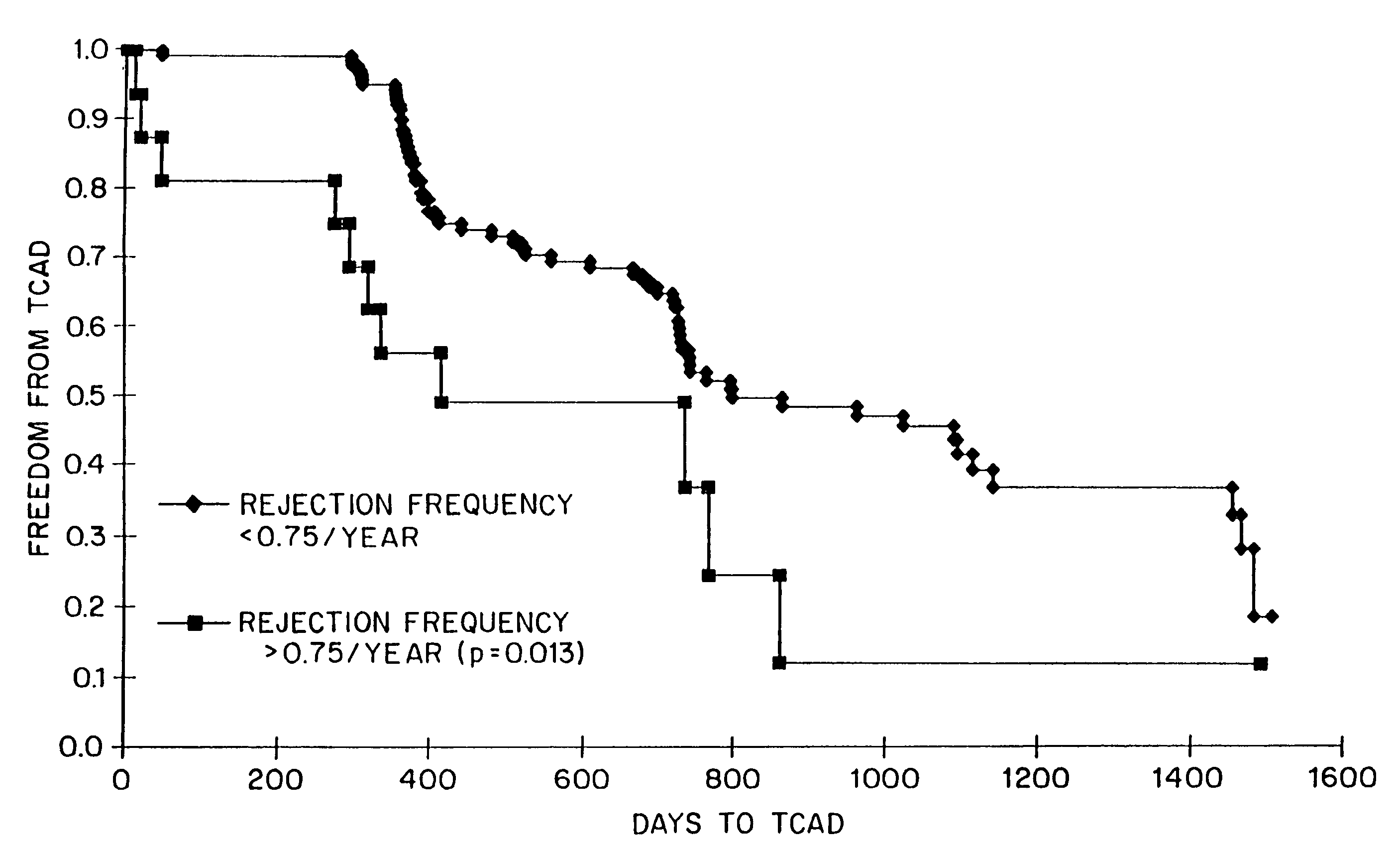
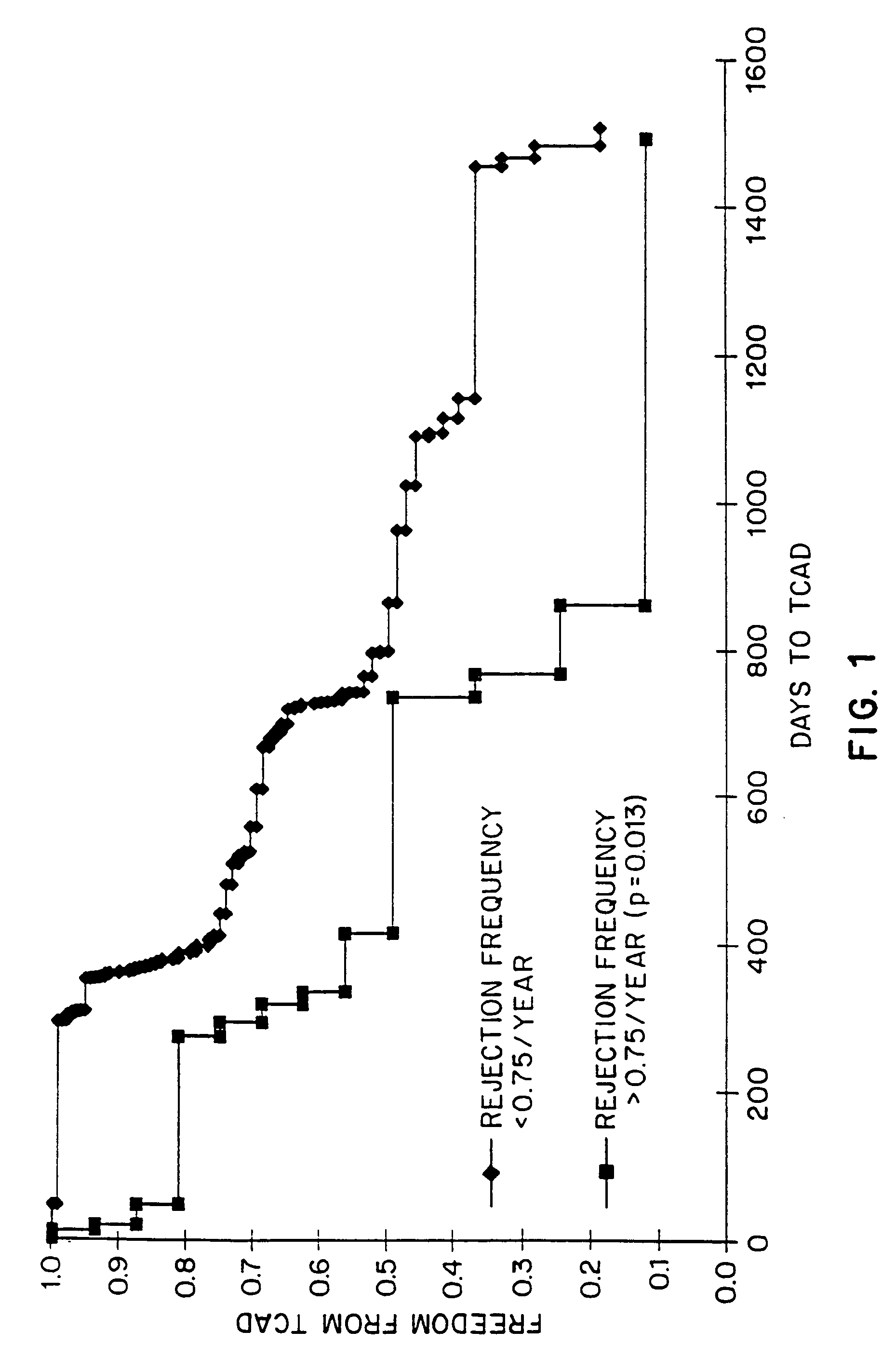
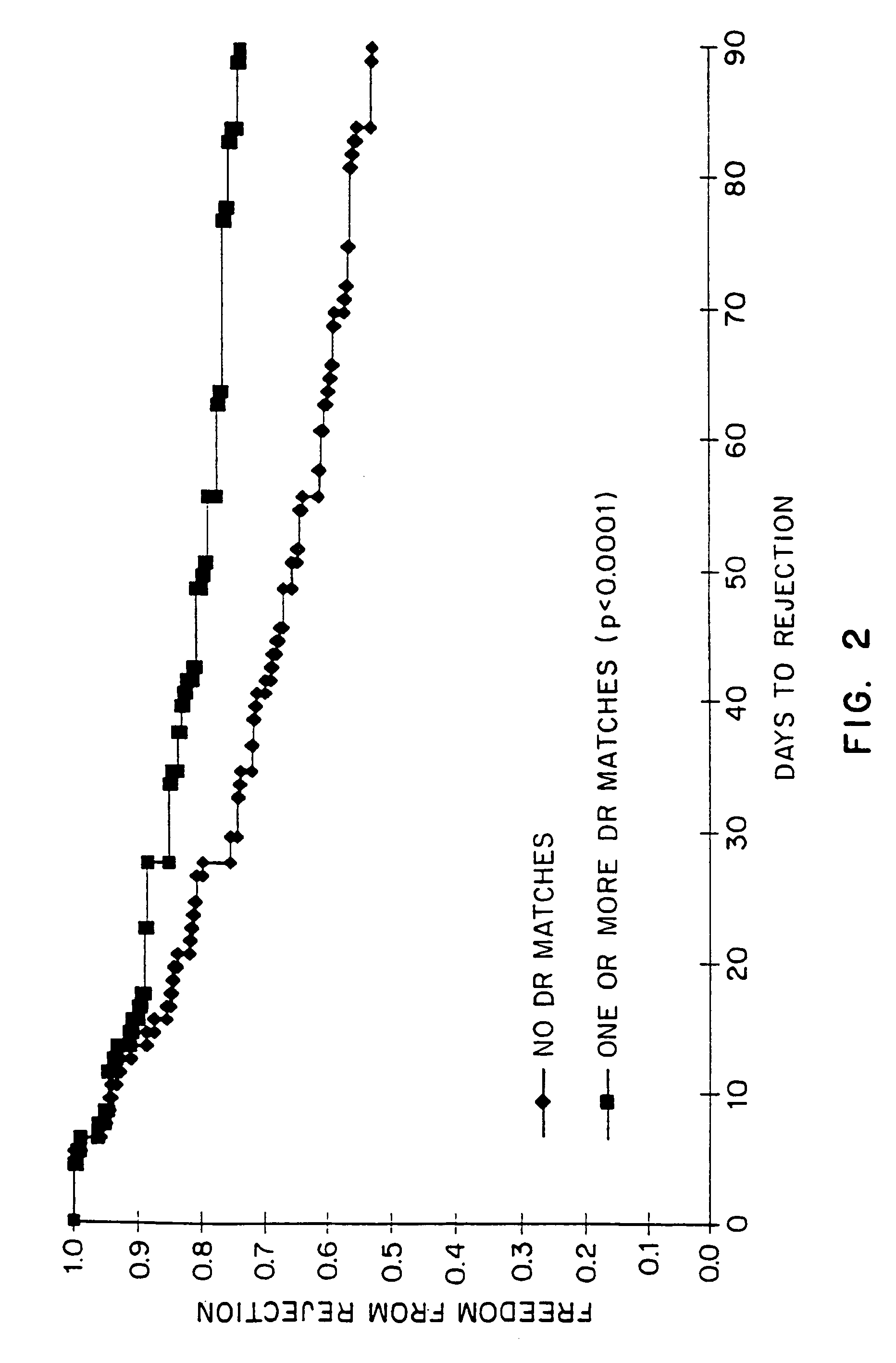
Kits for predicting transplant rejection
InactiveUS20060211035A1Reduces unnecessary diagnosticReduces therapeutic procedureMicrobiological testing/measurementBiological testingTransplant rejectionLower risk
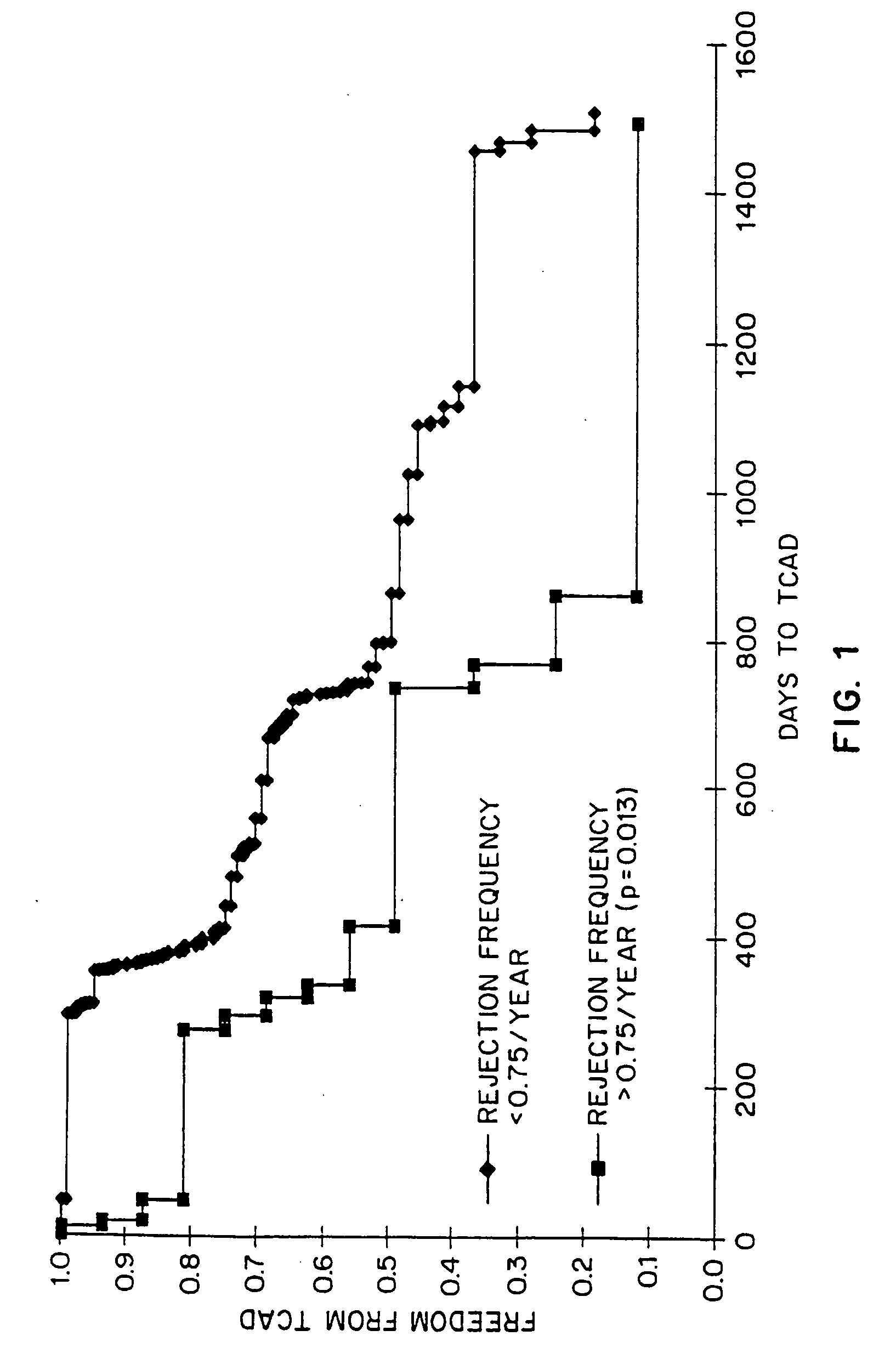
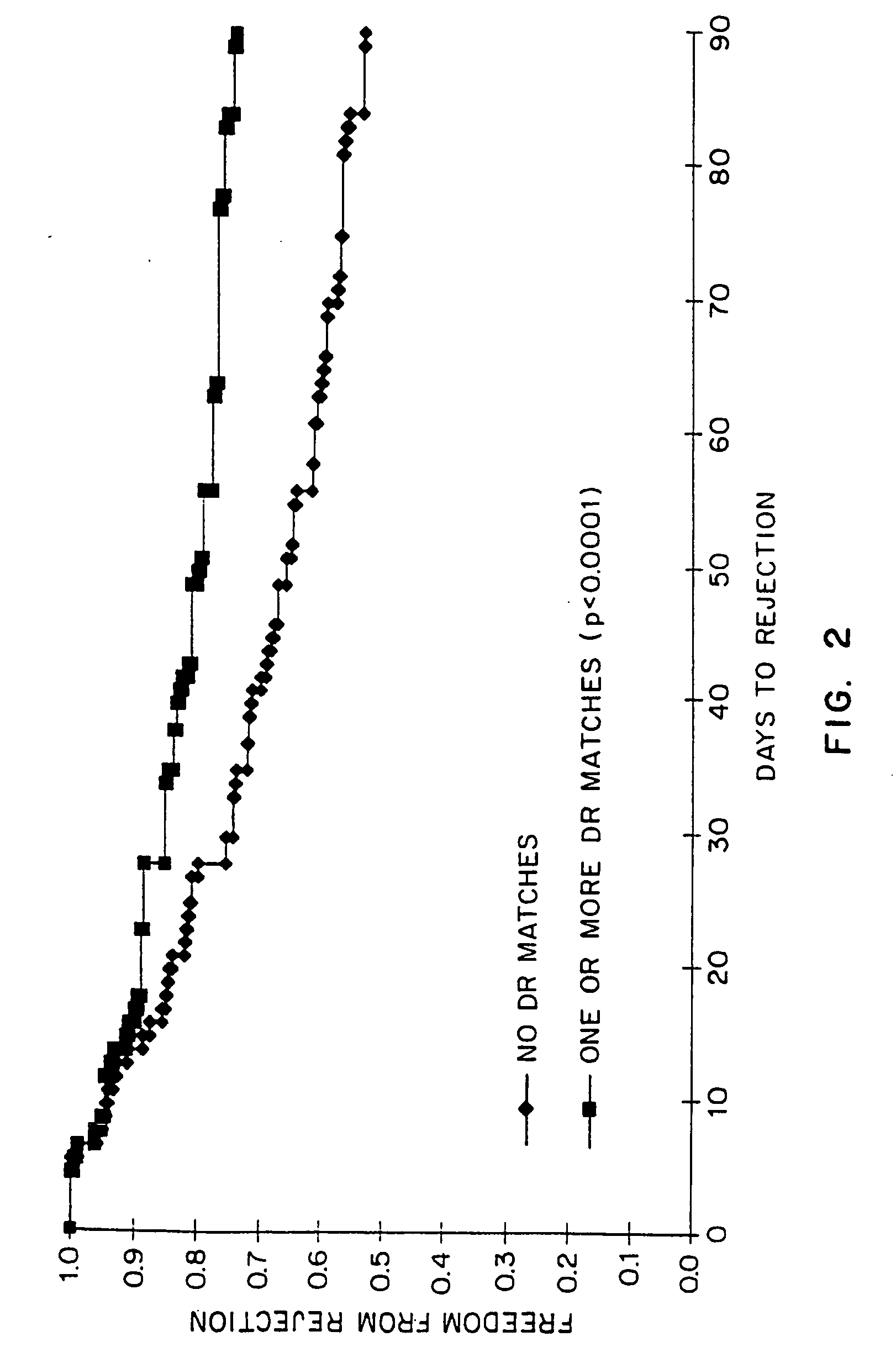
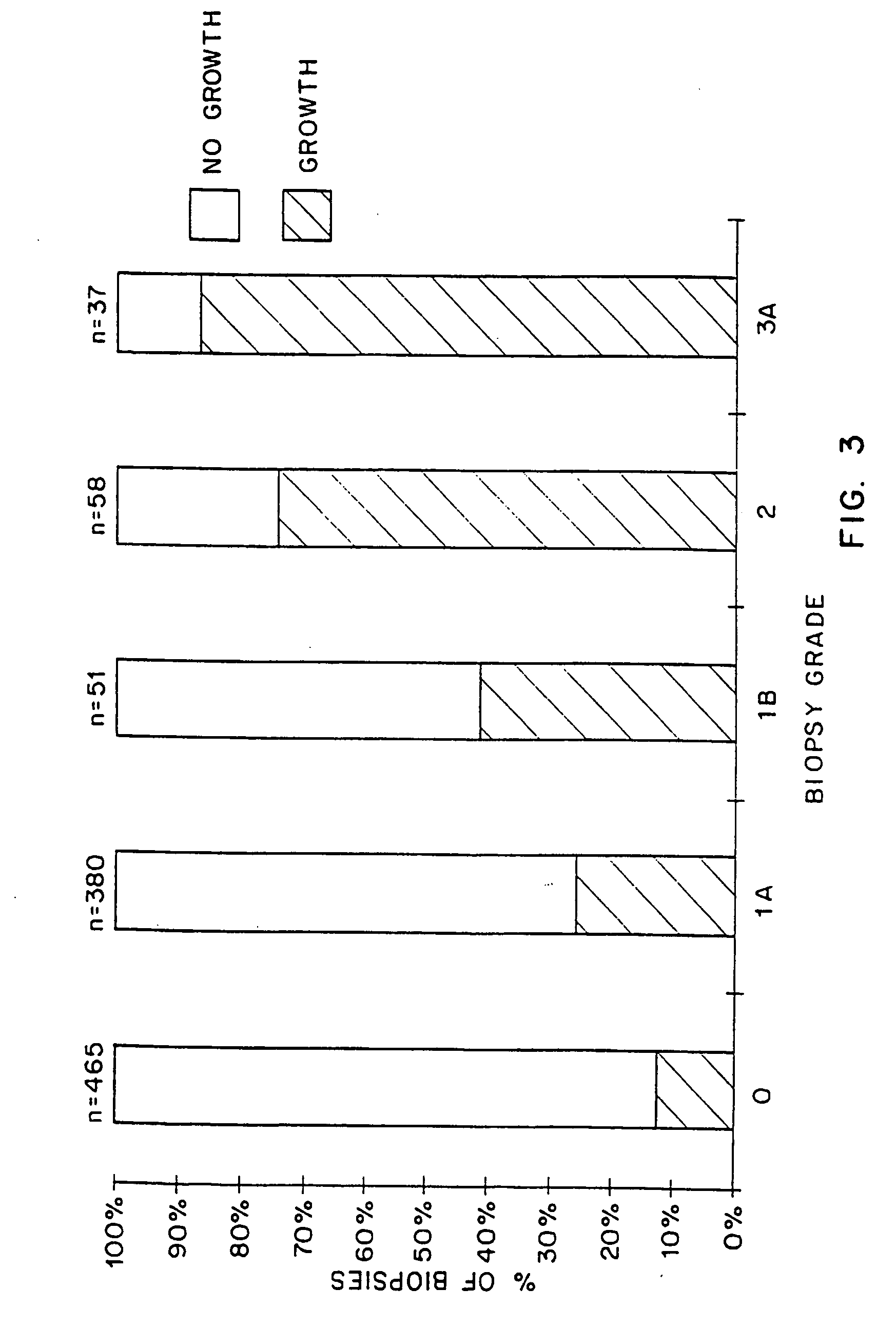
The present invention relates to a method for predicting whether or not transplant recipients are likely to reject tissue allografts. It is based, at least in part, on the discovery that based on analysis of three immunologic factors, cardiac transplant recipients could be classified into risk categories for progression to high-grade rejection. The present invention, by enabling a determination of the risk for high-risk rejection in a transplant patient, reduces unnecessary diagnostic and therapeutic procedures in low risk patients and clinical intervention in patients who would most benefit.
Owner:ITESCU SILVIU
Immunological reconstitution promoter or prophylactic agent for infections each of which maintains graft-versus-tumor effect
ActiveUS20120027748A1Good effectPromote infectionAntibacterial agentsAntimycoticsCvd riskWilms' tumor
The object of the invention is to provide an immunological reconstitution promoter or a prophylactic agent for infections for use in allogeneic hematopoietic stem cell transplantation therapy for tumors. The promoter or prophylactic agent enables the amelioration of delayed immune reconstitution or the prevention of infection following transplantation, while maintaining the GVT effect of allogeneic hematopoietic stem cell transplantation. Specifically, in a transplant patient in whom immune reconstitution is delayed, such reconstitution can be promoted by administering, at an early stage following transplantation, a substance capable of depleting CD4+ cells. Early completion of infection management in the patient and improvement in the survival rate are anticipated as a result. In addition, the risk of complications associated with allogeneic hematopoietic stem cell transplantation is reduced, enabling more widespread use of this therapy.
Owner:KOUJI MATSUSHIMA
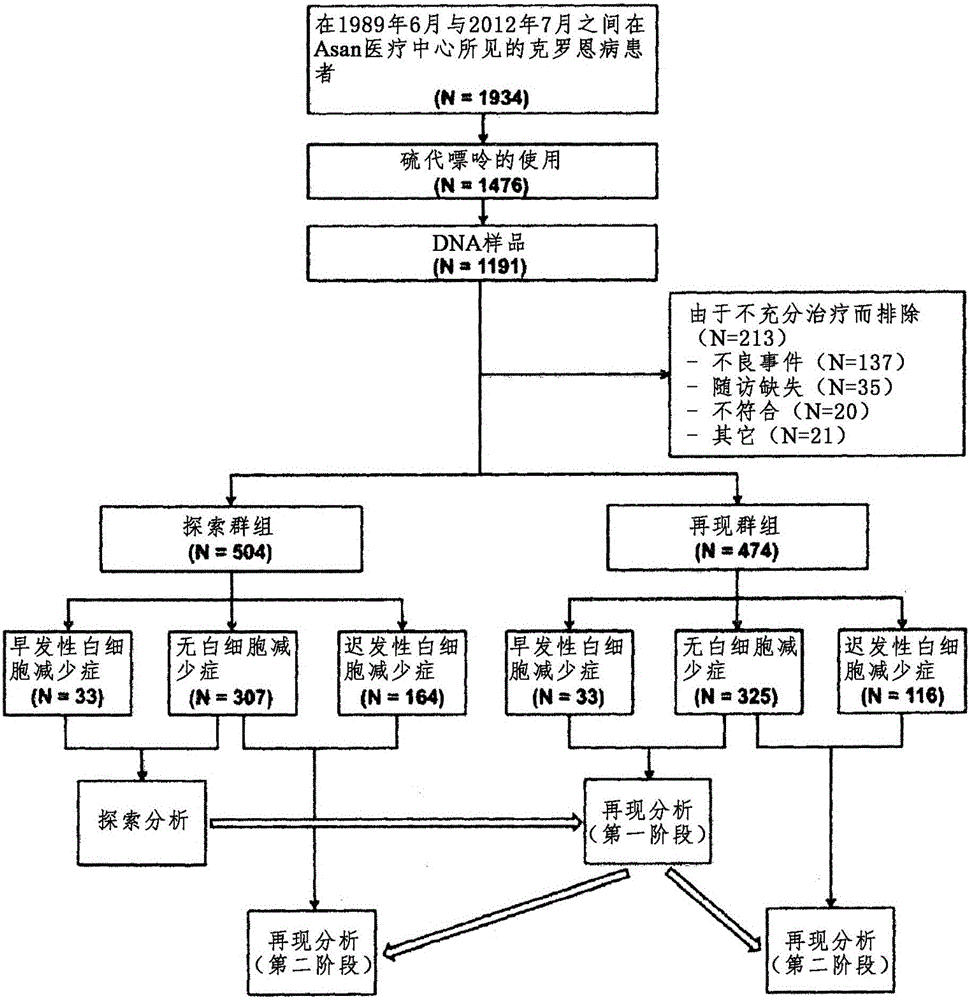
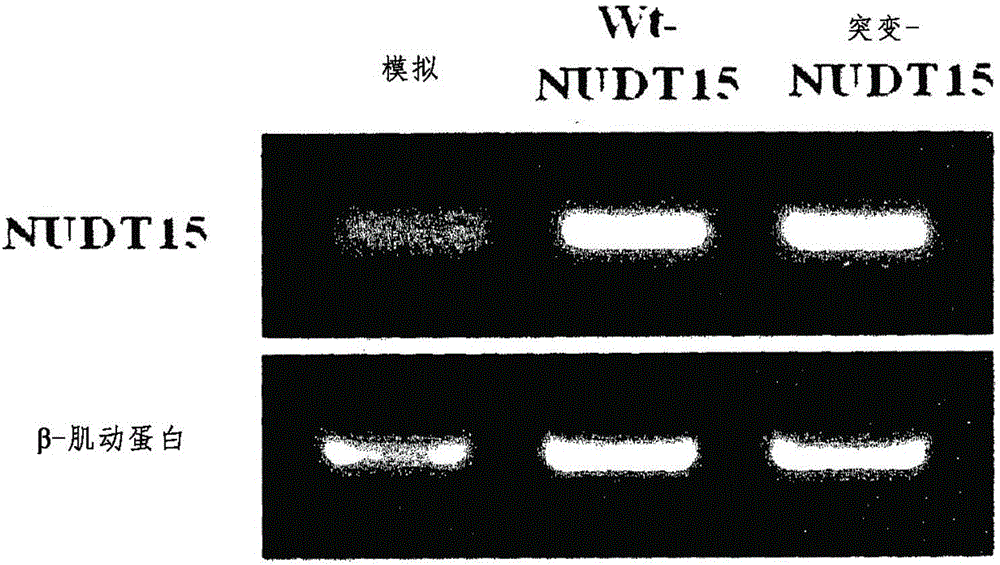
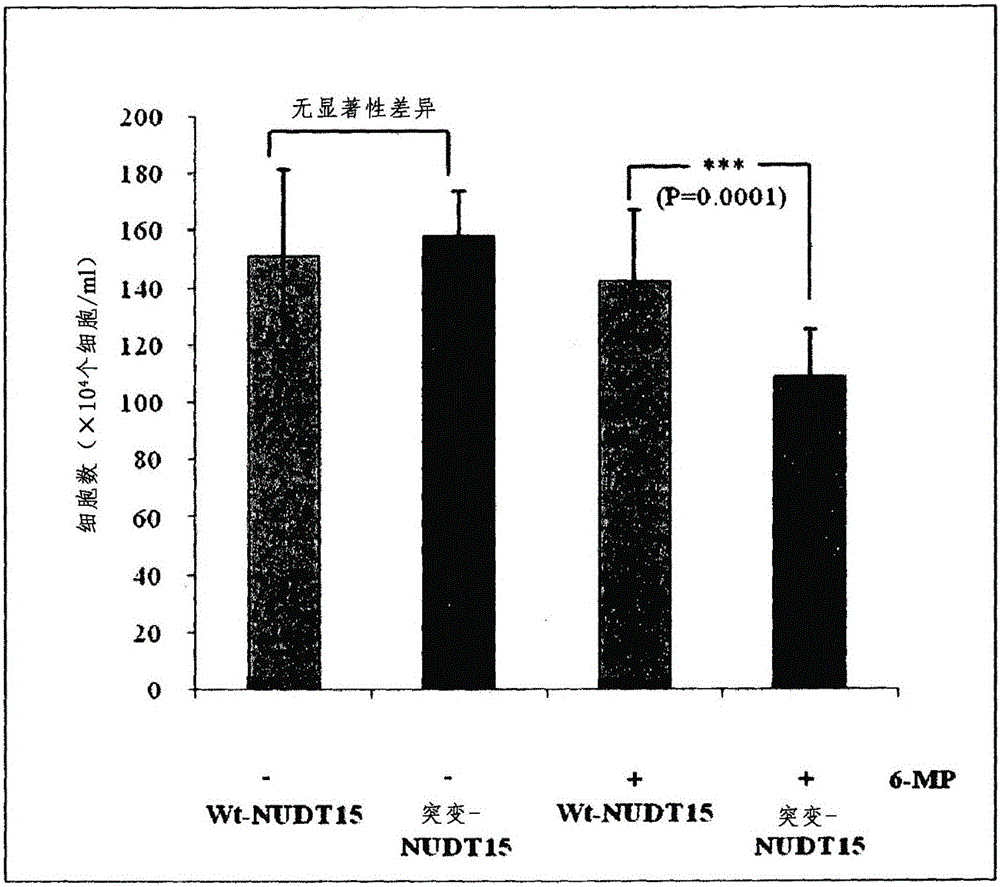
Composition for predicting risk of thiopurine-induced leukopenia, containing single nucleotide polymorphism marker within NUDT15 gene
ActiveCN106536749AImplement individualized treatmentMicrobiological testing/measurementDNA/RNA fragmentationCrohn's diseaseUlcerative colitis
The present invention relates to a composition for predicting the risk of thiopurine-induced leukopenia, containing a single nucleotide polymorphism marker within NUDT15 gene, and specifically, to: a composition for predicting the risk of thiopurine-induced leukopenia in Crohn's disease, ulcerative colitis, leukemia or transplant patients, containing a single nucleotide polymorphism marker within NUDT15 gene; a kit for predicting the risk; and a method for predicting the risk. According to the present invention, a patient having high sensitivity to leukopenia, which occurs during thiopurine therapy, in Crohn's disease, ulcerative colitis, leukemia or transplant patients can be rapidly classified early on, and thus patient-customized therapy can be efficiently carried out.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION +1
Method for predicting transplant rejection
InactiveUS7135302B1Reduces unnecessary diagnosticReduces therapeutic procedureMicrobiological testing/measurementBiological testingTransplant rejectionLower risk
The present invention relates to a method for predicting whether or not transplant recipients are likely to reject tissue allografts. It is based, at least in part, on the discovery that, based on analysis of three immunologic factors, cardiac transplant recipients could be classified into risk categories for progression to high-grade rejection. The present invention, by enabling a determination of the risk for high-risk rejection in a transplant patient, reduces unnecessary diagnostic and therapeutic procedures in low risk patients and clinical intervention in patients who would most benefit.
Owner:ITESCU SILVIU
Tissue management system
InactiveUS20140297325A1Mechanical/radiation/invasive therapiesComputer-assisted treatment prescription/deliveryManagement systemMedical treatment
The present invention provides a comprehensive tissue management system for transplantable materials like tissues and organs. The tracking portion of the system prompts and verifies that staff members of a medical establishment like a hospital have handled, stored, transported, reconstituted, and used the tissue or organ materials in a safe and regulatory-compliant manner from the point of receipt to the point of issuance or surgical use throughout the hospital's organization. The tracing portion of the system creates an integral record that documents which hospital staff members have provided which processing steps to the tissue or organ, any associated materials used in conjunction with such tissue or organ, and an identification of the tissue or organ that was transplanted or implanted inside a patient. Such a system will enable adverse reaction investigations for transplant patients, and recalls of transplantable materials.
Owner:BIOMEDICAL SYNERGIES
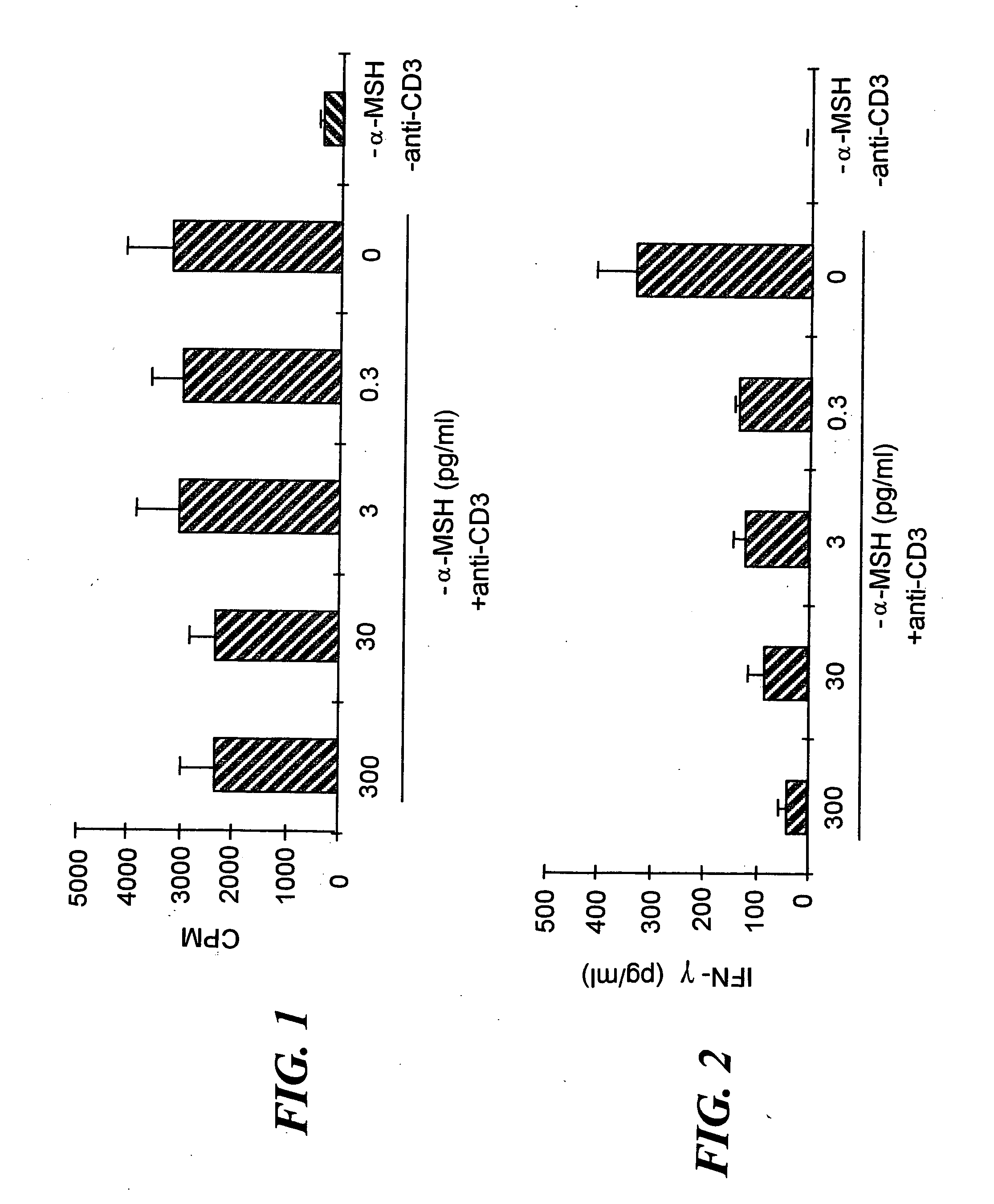
Activation of regulatory T cells by alpha-melanocyte stimulating hormone
InactiveUS20060127400A1Reduce riskInhibit inflammationPeptide/protein ingredientsAntipyreticTolerabilityAutoimmune condition
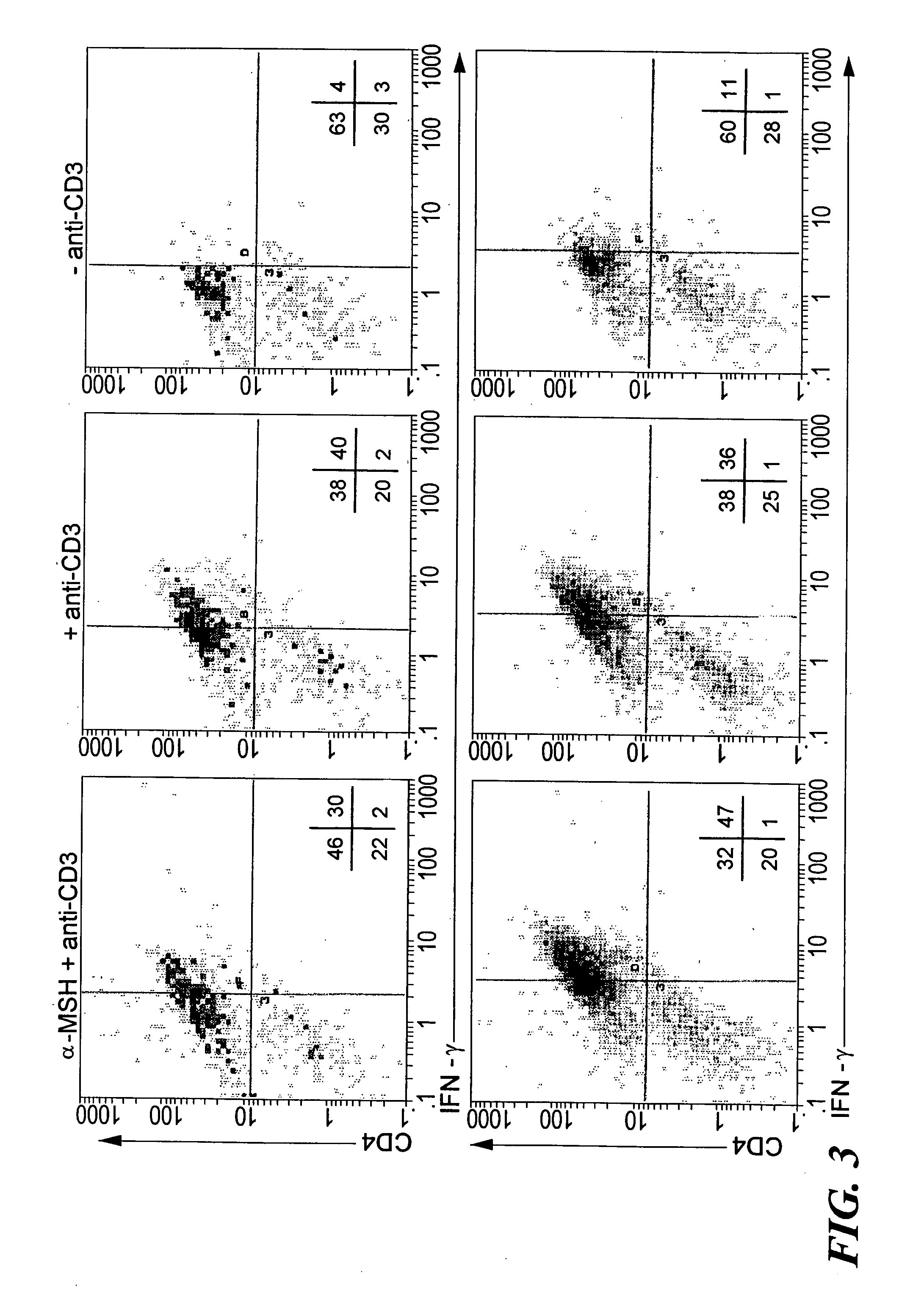
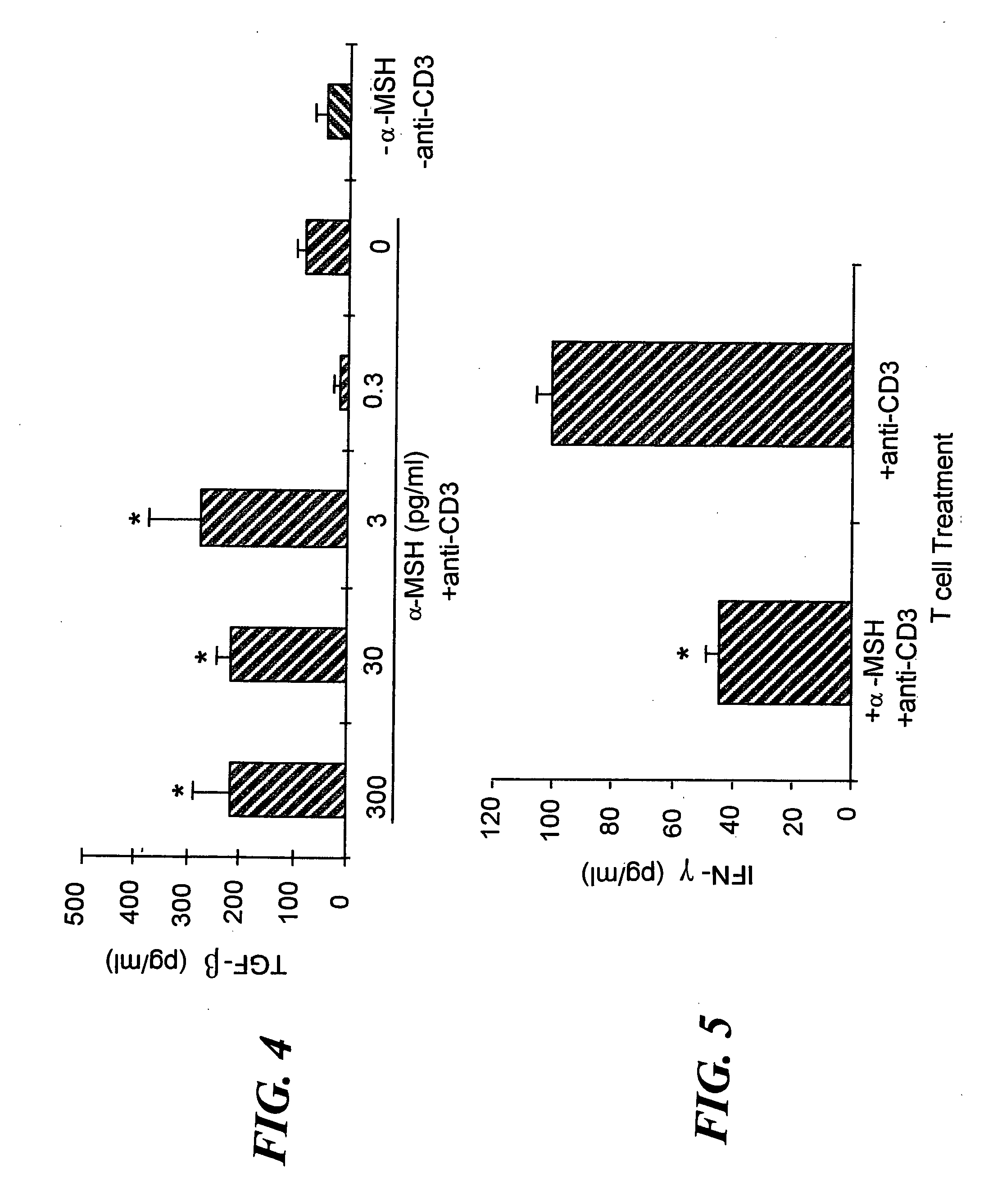
The invention encompasses a method of down-regulating a T cell-mediated immune response, through activation or T cell receptor (TCR) stimulation of antigen-primed T cells in the presence of alpha-melanocyte stimulating hormone (α-MSH), which may be optionally enhanced by adding transforming growth factor-β2 (TGF-β2) approximately 4-6 hours after the start of the primed T cells' exposure to α-MSH. Activation of the primed T cells may be mediated by presentation of the specific antigen to the primed T cells, or by an anti-TCR antibody or a T cell mitogen. As a result of the α-MSH treatment modulating the T cell activation, antigen-specific, regulatory, CD4+ / CD25+ T cells are generated that produce transforming growth factor-β (TGF-β) and can non-specifically down-regulate Th1-mediated inflammatory activities. The method may be used to down-regulate or suppress an autoimmune condition or a graft rejection in a transplant patient. The invention also encompasses a kit for generating regulatory T cell comprising a specific antigen, α-MSH, and optionally, TGF-β2 and / or a T cell culture medium. Also provided are gene therapy treatments for suppressing an autoimmune or graft rejection response, or for re-establishing autotolerance, by introducing genetic material (e.g. nucleic acid) for expressing α-MSH or a receptor-binding portion thereof, into a localized tissue site.
Owner:THE SCHEPENS EYE RES INST
Suppressor of the endogenous interferon-gamma
The invention relates to suppressors of endogenous human interferon-gamma (INF-γ) applicable in treatment of diseases associated with impaired activity of endogenous IFN-γ. The suppressors of the invention are useful in treating autoimmune diseases and for prevention of graft arteriosclerosis and rejection of organs in allograft transplanted patients. The invention includes inactive analogues or variants of IFN-γ having preserved affinity to the IFN-γ receptor, genetically modified in the domain responsible for triggering the signal transduction pathway.
Owner:MILLICOM
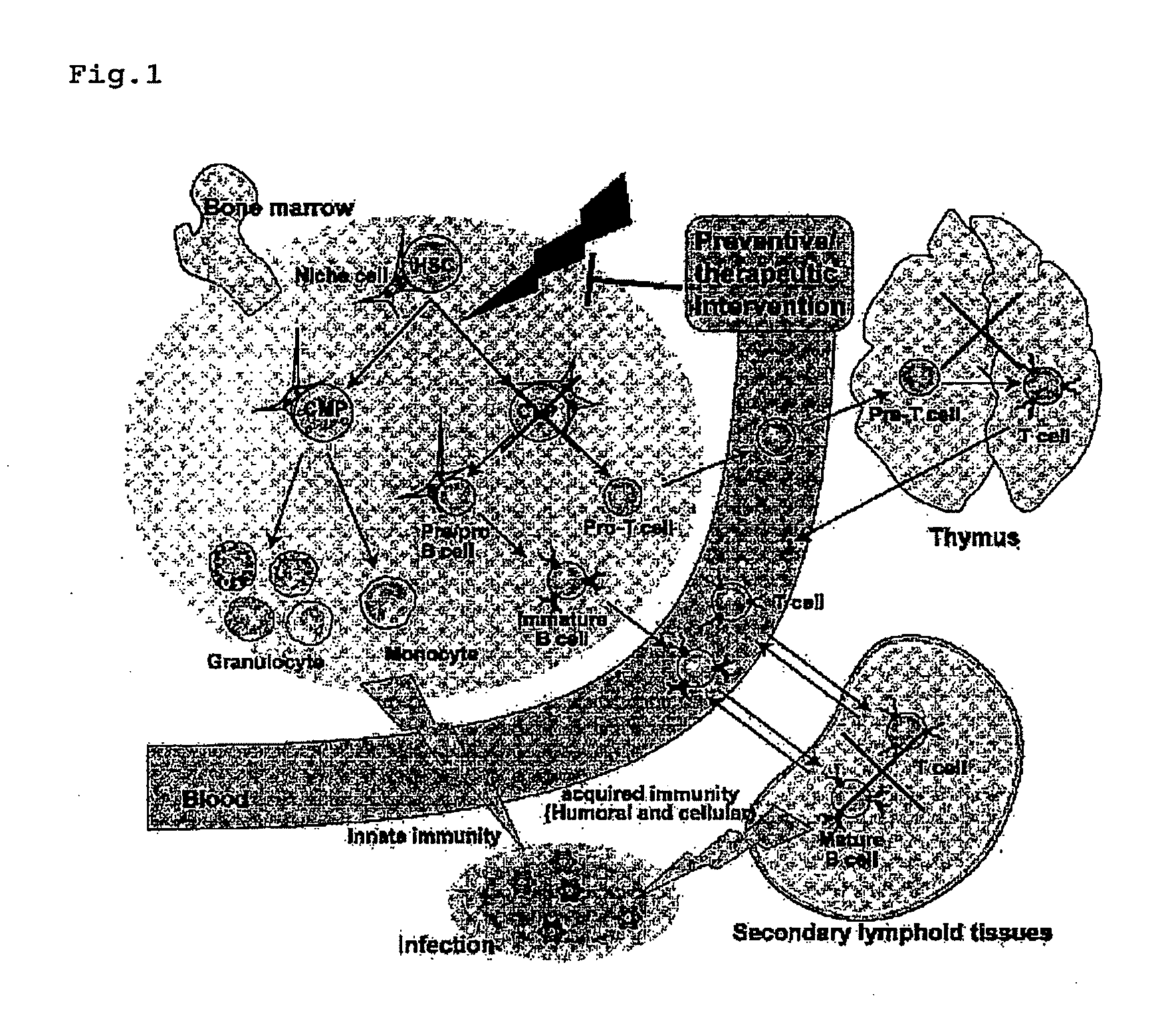
Improved cell therapy compositions for hematopoietic stem cell transplant patients
PendingUS20210213066A1Highly personalized therapyImprove responseViral antigen ingredientsMammal material medical ingredientsHematopoietic stem cell transplantationT cell
The present disclosure provides for isolated and processed cell therapeutic compositions and Methods of using those compositions for the treatment of a patient undergoing a hematopoietic stem cell transplant (HSCT). In some embodiments, the disclosure provides for methods of making these cells by exposing the isolated T cell populations to one or more tumor antigens.
Owner:CHILDRENS NAT MEDICAL CENT
Induction of immunosuppression by inhibition of ATM
InactiveUS20080279866A1Prevent rejectionImpaired to respondOrganic active ingredientsMetabolism disorderAutoimmune responsesAllergy
The present invention is directed to methods of inducing immunosuppression in a patient by administering an inhibitor of the enzyme Ataxia telangiectasia mutated (Atm). The method may be used as a treatment for allergies, autoimmune diseases or lymphomas. It may also be used to prevent organ rejection in transplant patients and to treat or prevent graft versus host disease.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Methods for identifying patients at risk for costimulation blockade resistant rejection
InactiveUS20160153990A1Microbiological testing/measurementDisease diagnosisCostimulation blockadeT cell
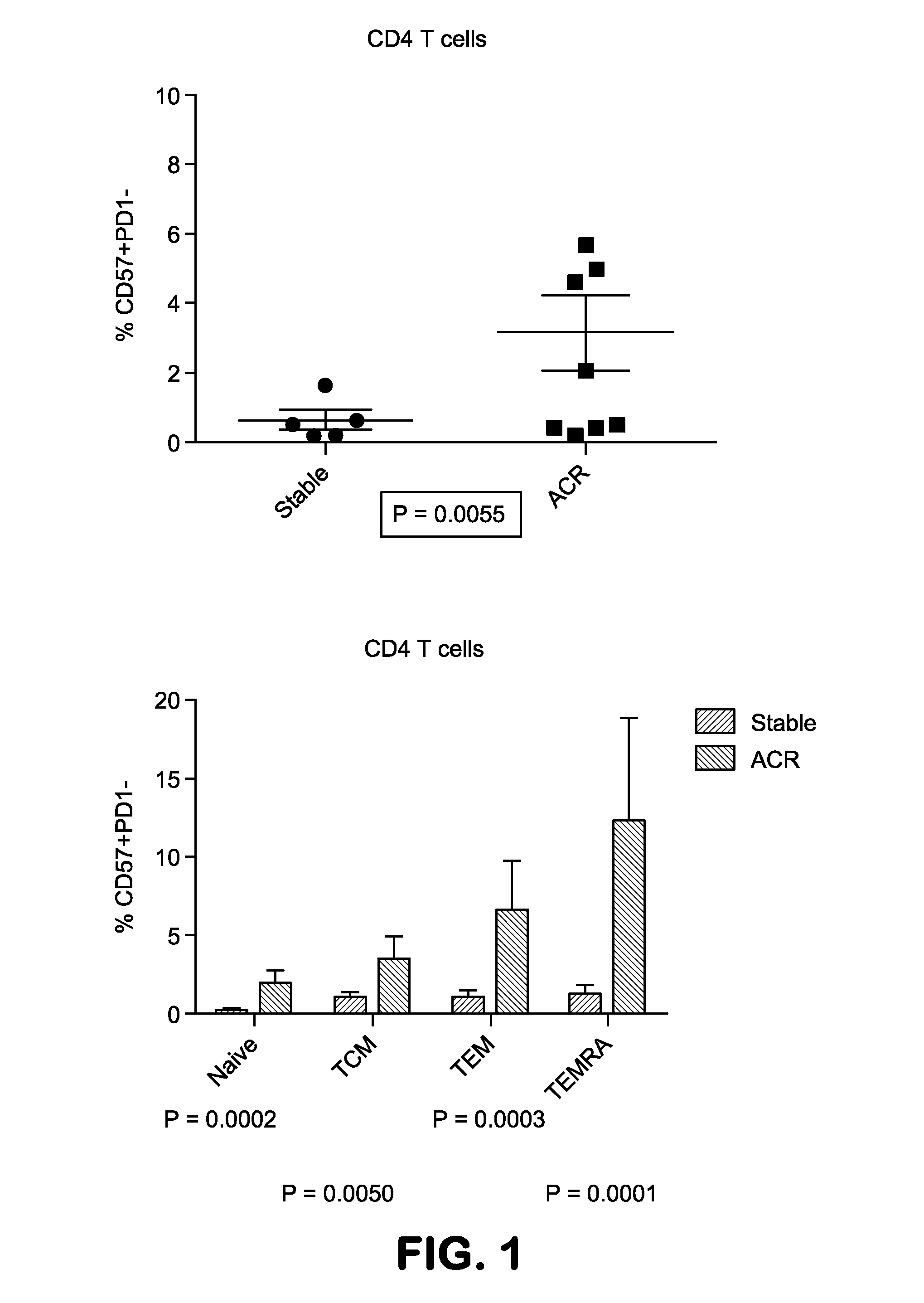
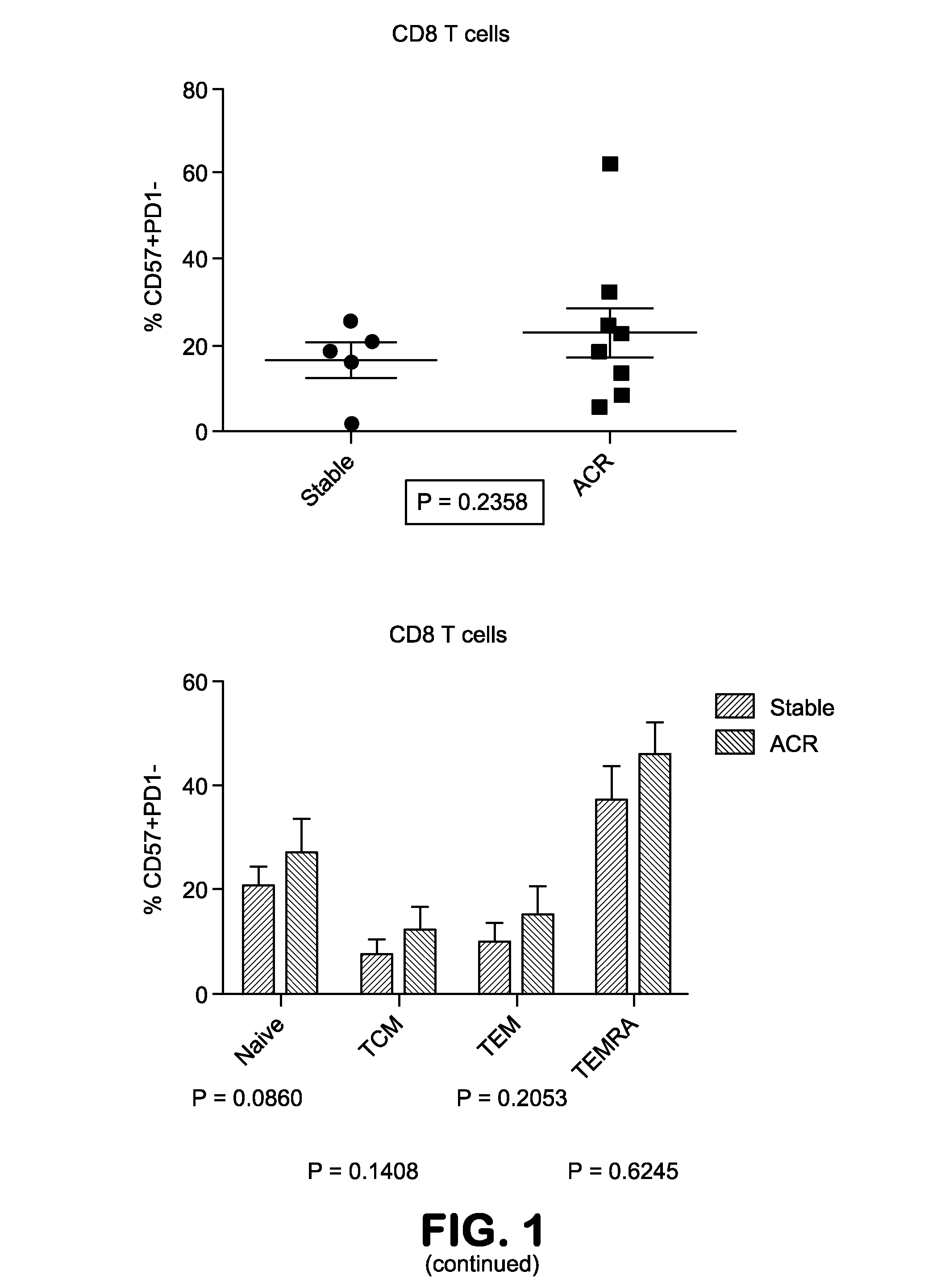
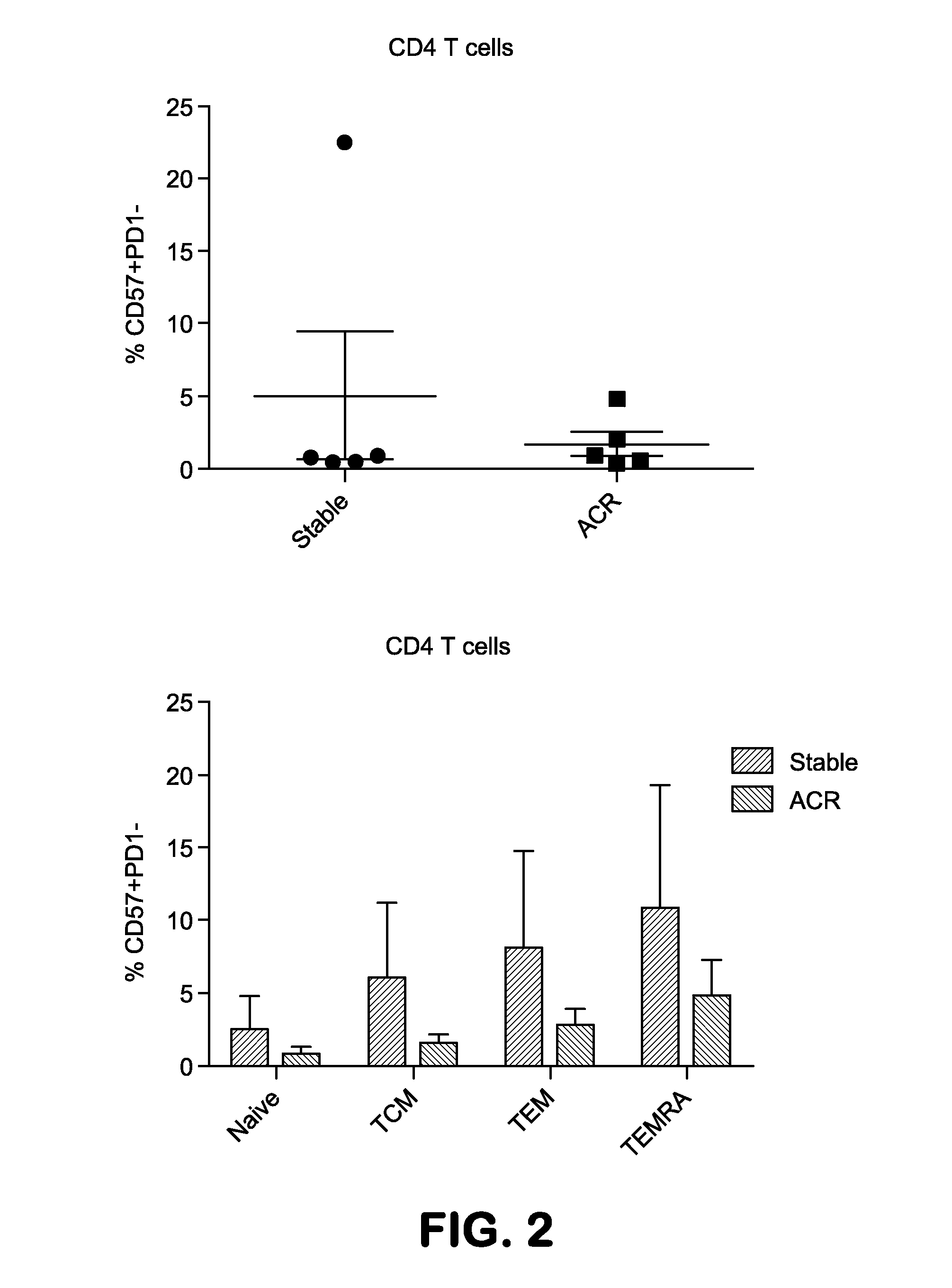
The present invention provides methods utilizing changes in CD4+CD57+ T cells levels for determining the susceptibility of a transplant patient or patient in need thereof to costimulation blockade resistant rejection. These methods are useful for identifying effective drug regimens for the treatment of immune disorders associated with graft transplantation and / or maintenance of a transplant.
Owner:BRISTOL MYERS SQUIBB CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com