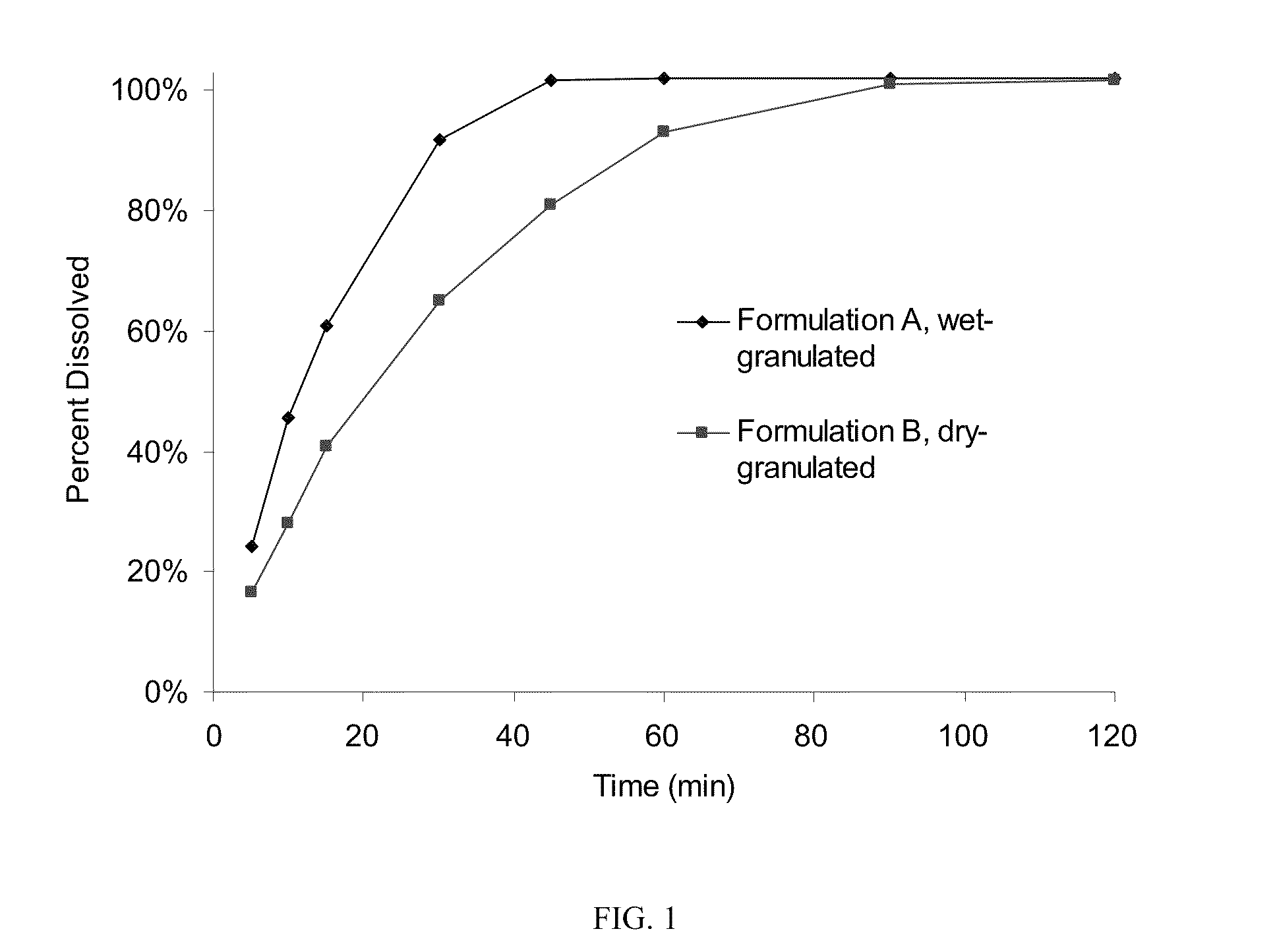
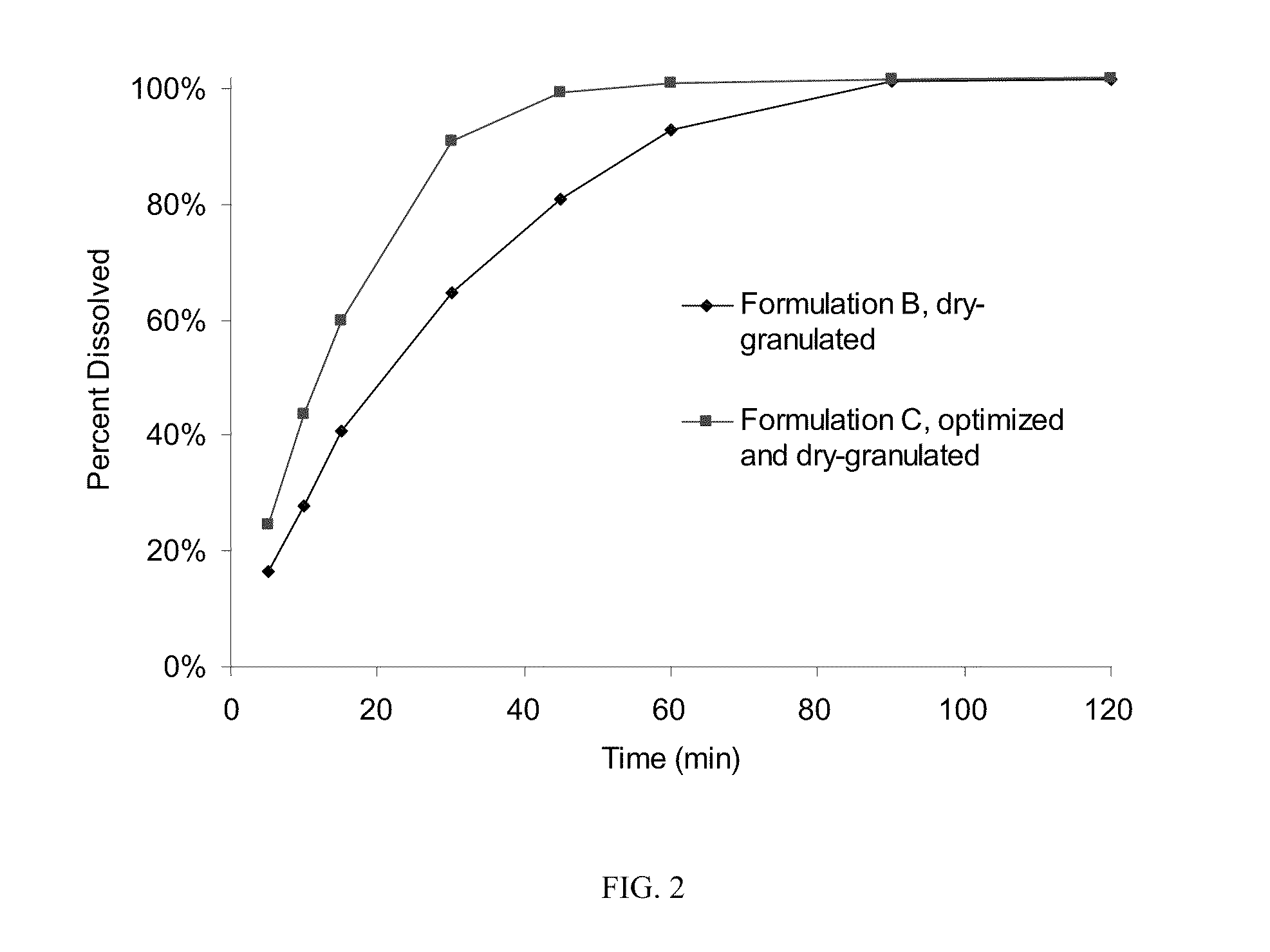
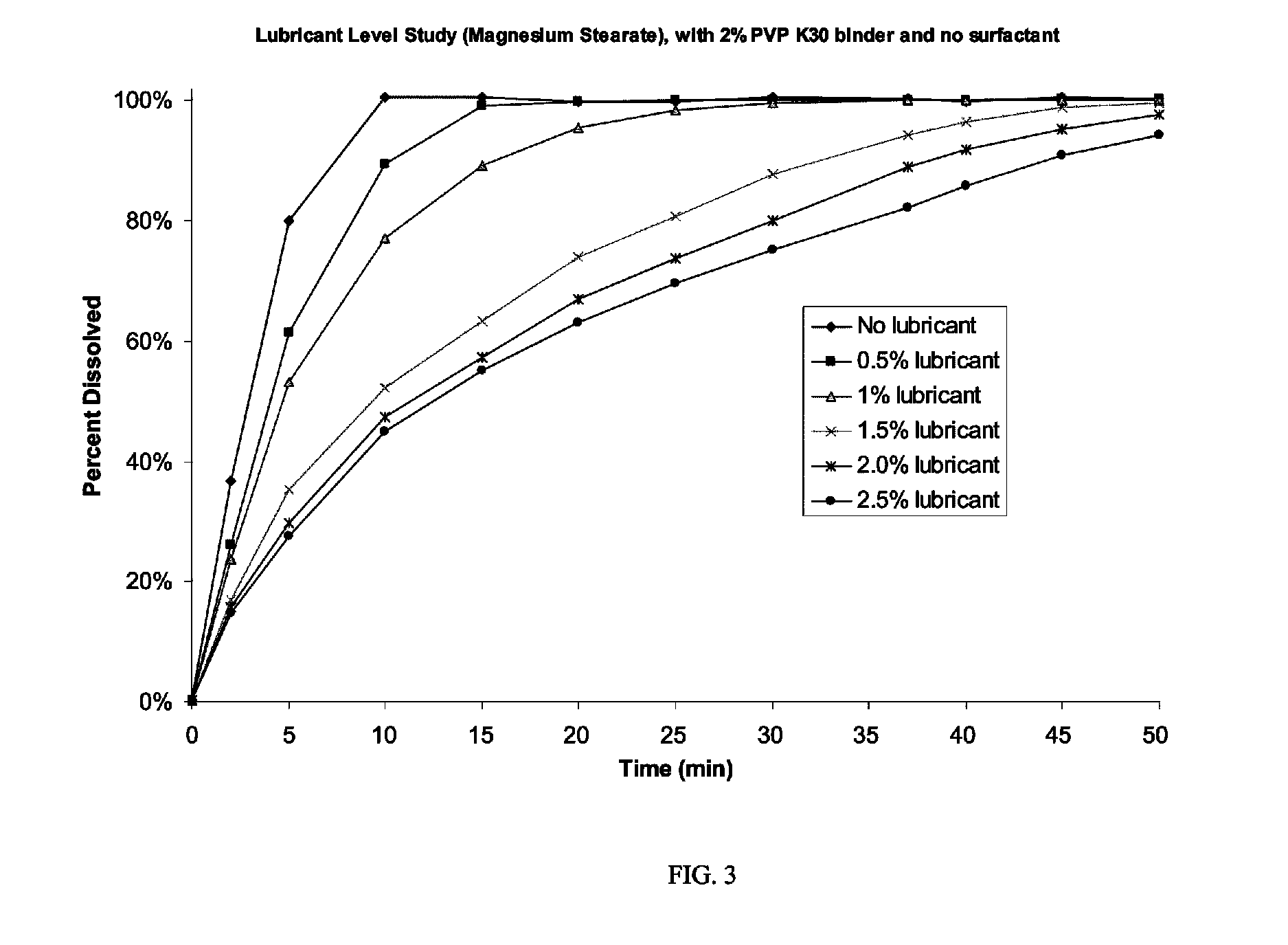
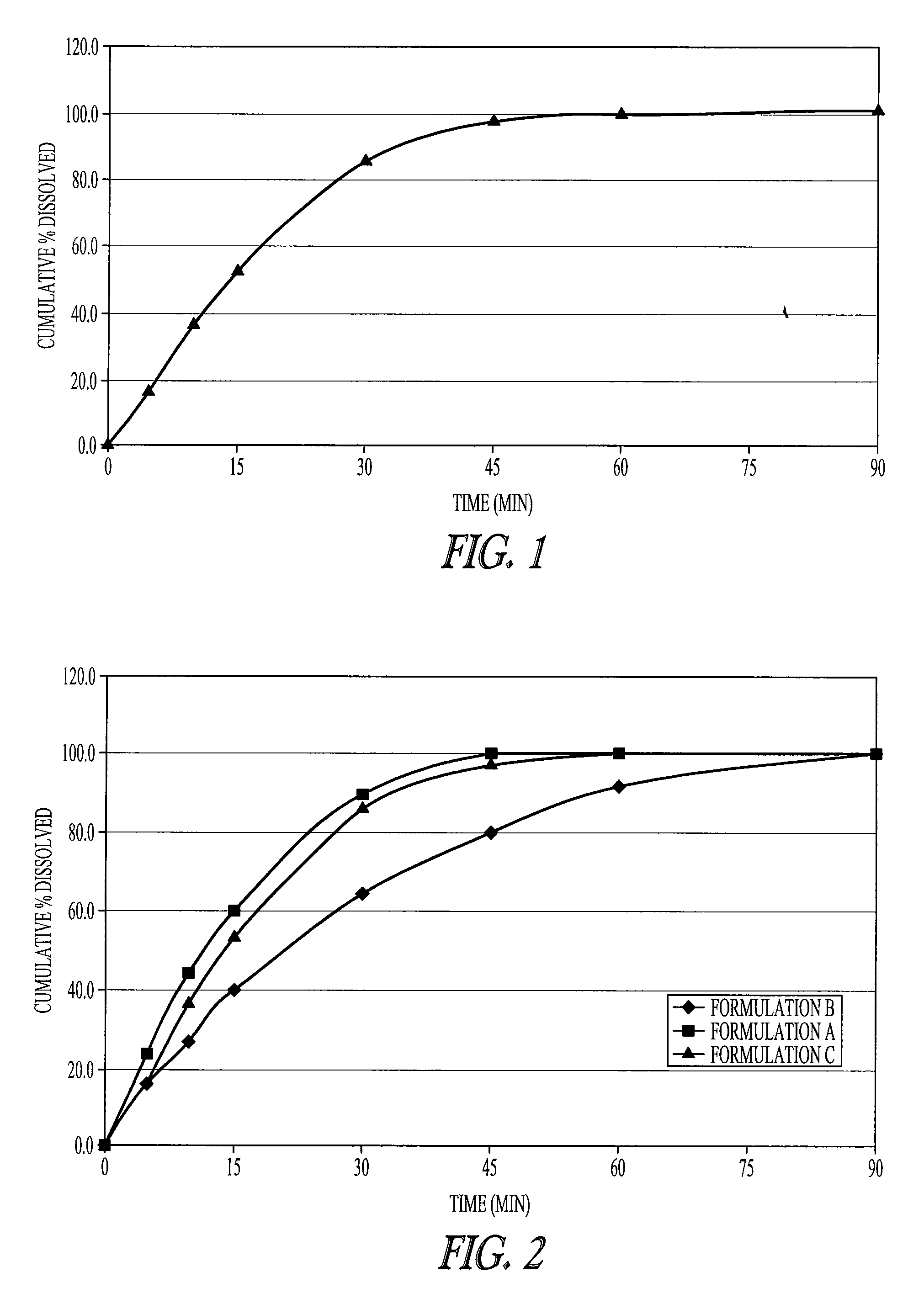
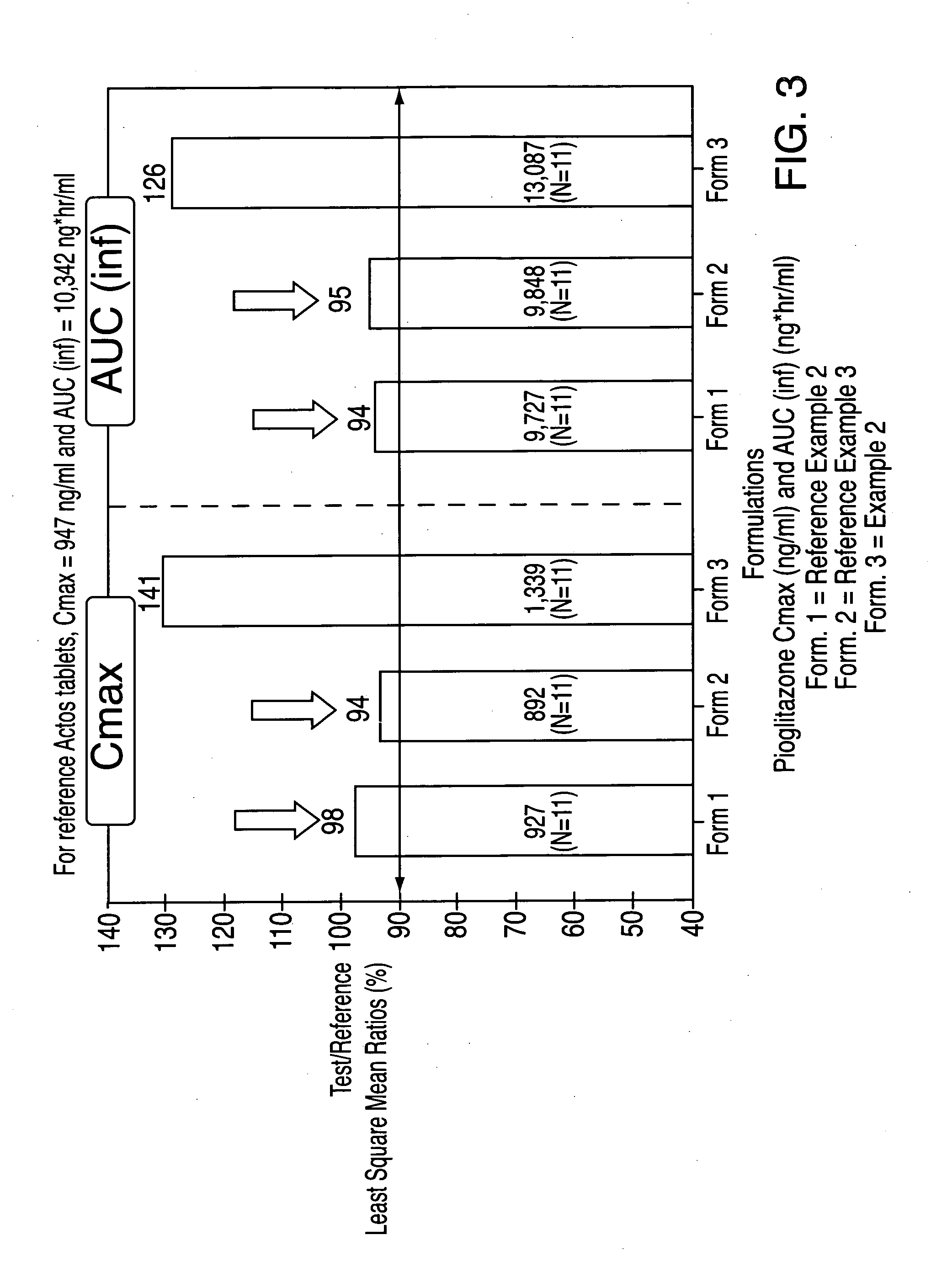
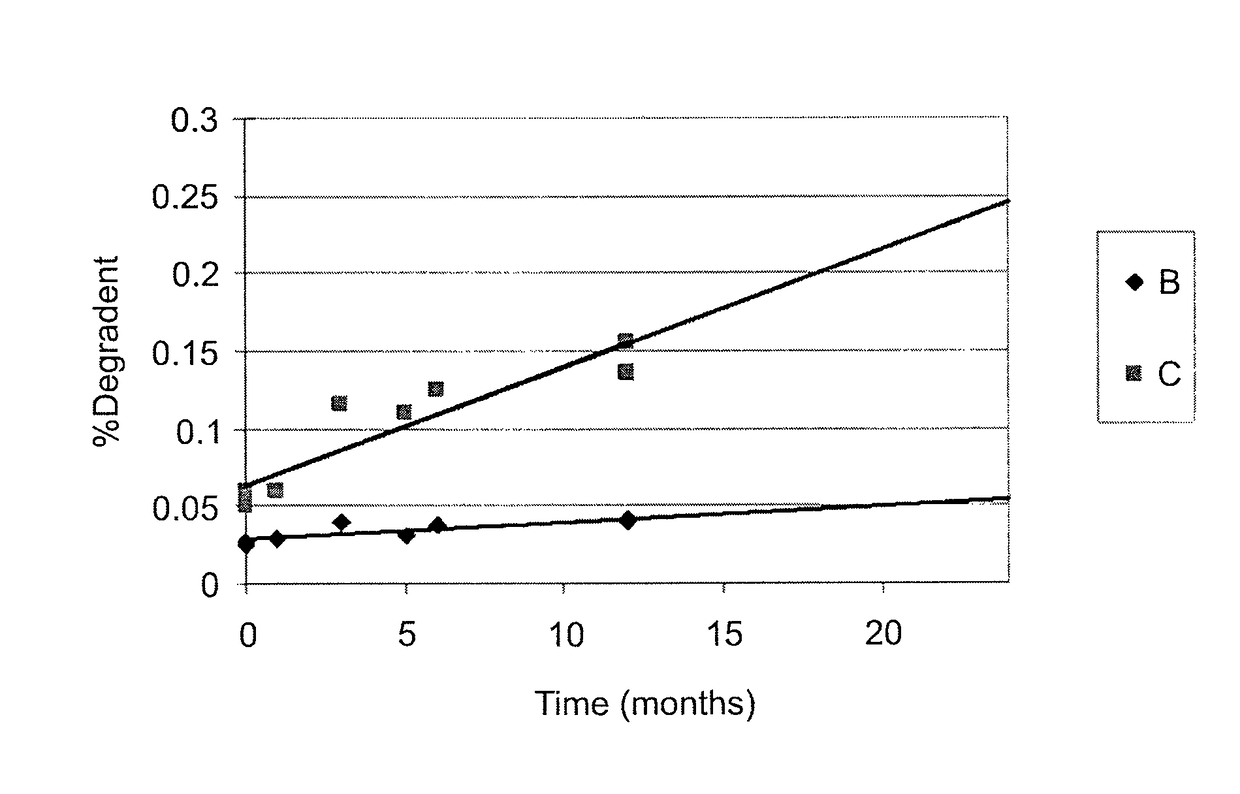
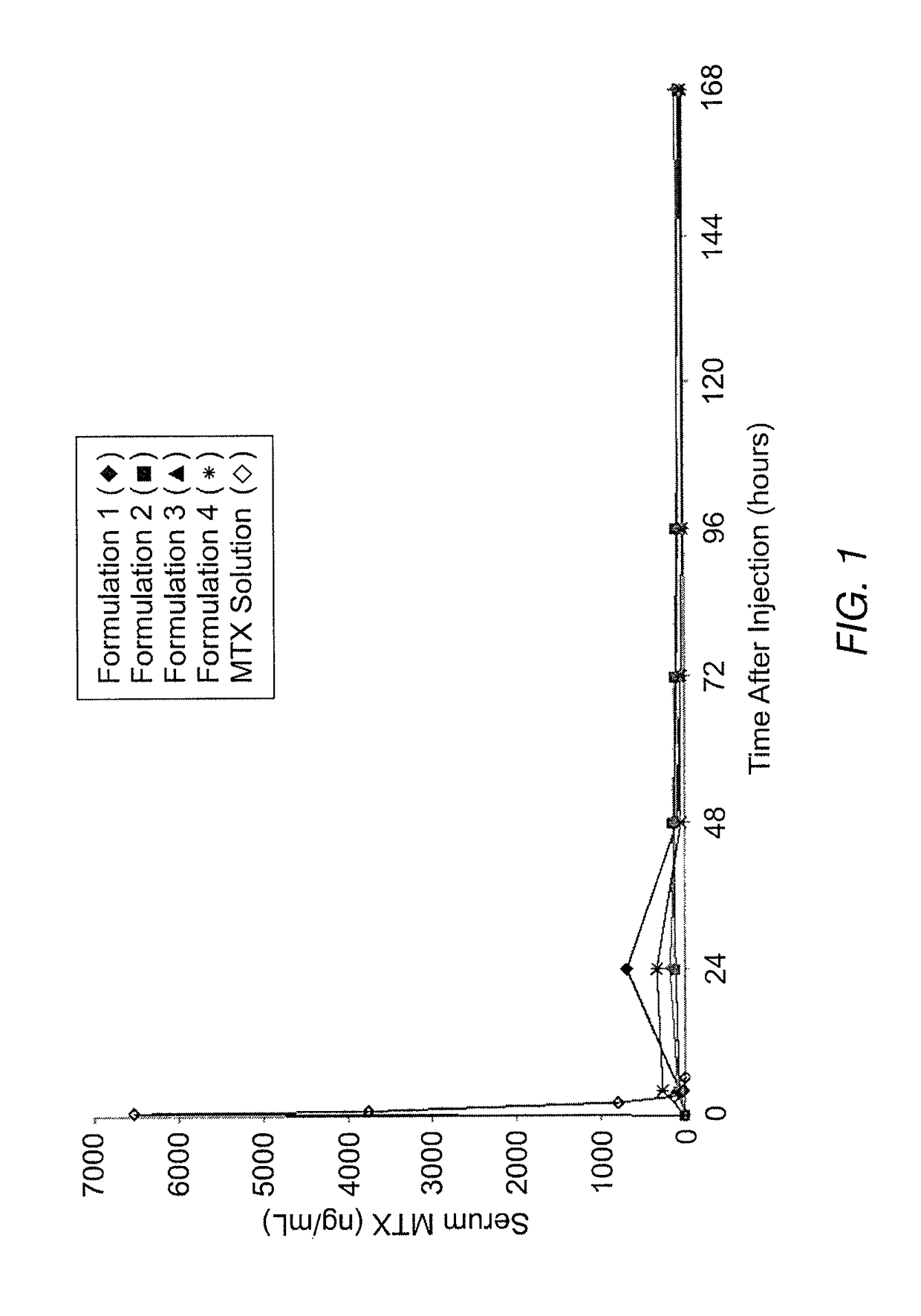
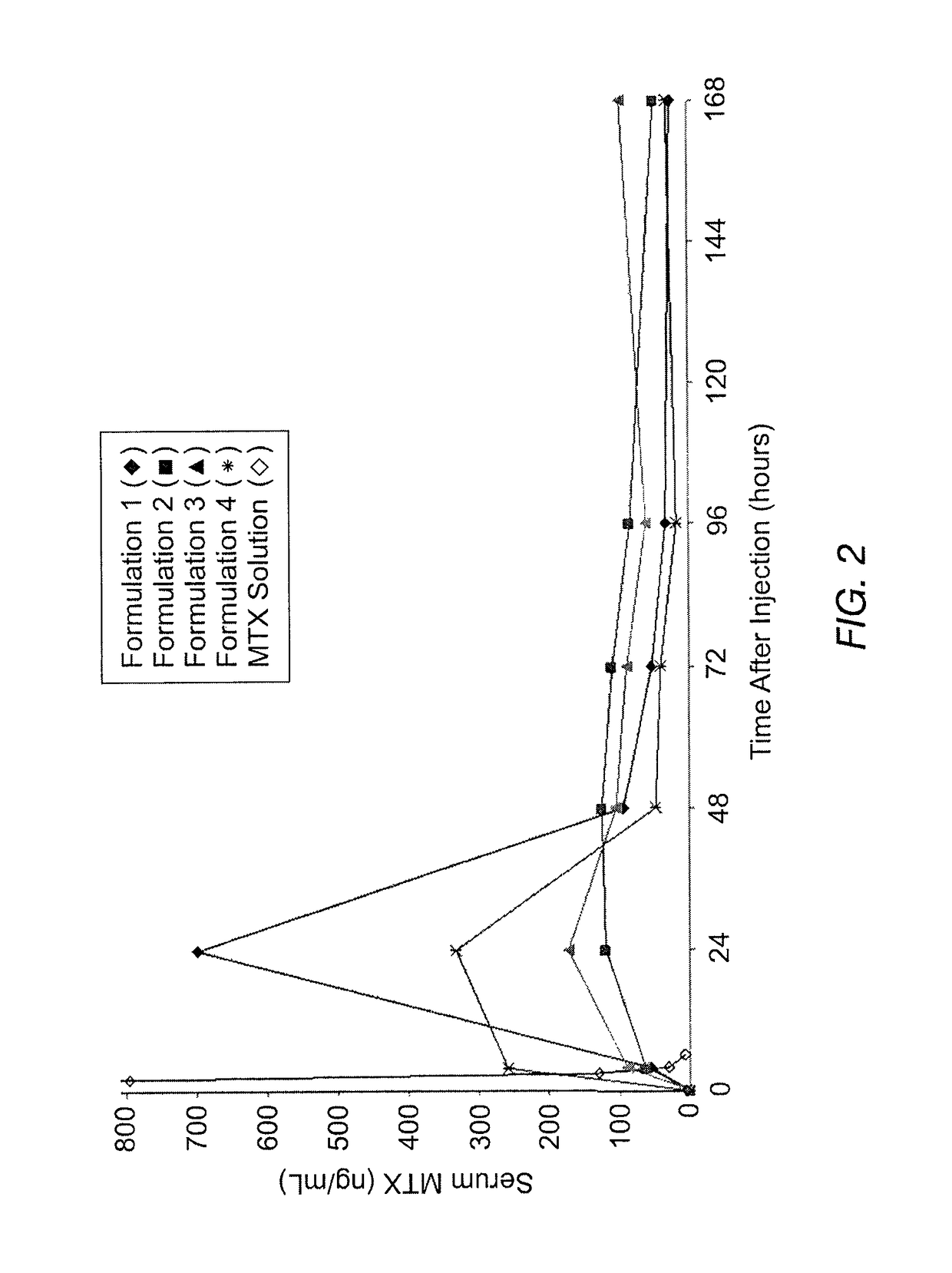
Patents
Literature
34 results about "Immediate Release Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A solid, semi-solid, solution or suspension that is designed to release its active and/or inert ingredient(s) upon administration with no enhanced, delayed or extended release effect.
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
Immediate release formulations and dosage forms of gamma-hydroxybutyrate
The present invention provides a solid immediate release dosage form adapted for oral administration of GHB. The solid immediate release dosage form includes an immediate release formulation comprising a relatively high weight-percentage of GHB with a bioavailability similar to that of a liquid GHB dosage form.
Owner:JAZZ PHARMA INC
Immediate release dosage forms of sodium oxybate
The present invention provides a pharmaceutical composition, presented as a solid unit dosage form adapted for oral administration of sodium oxybate. The preferred unit dosage form is a tablet comprising a relatively high weight-percentage of sodium oxybate, in combination with a relatively small weight-percentage of total excipients. This permits the tablets to contain / deliver a pharmaceutically effective amount, e.g., about 0.5-1.5 g of sodium oxybate in each tablet with a delivery profile similar to that of the liquid form. The tablets are bioequivalent to the liquid form.
Owner:JAZZ PHARMA
Methods and dosage forms for controlled delivery of paliperidone and risperidone
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Immediate release dosage forms of sodium oxybate
The present invention provides a pharmaceutical composition, presented as a solid unit dosage form adapted for oral administration of sodium oxybate. The preferred unit dosage form is a tablet comprising a relatively high weight-percentage of sodium oxybate, in combination with a relatively small weight-percentage of total excipients. This permits the tablets to contain / deliver a pharmaceutically effective amount, e.g., about 0.5-1.5 g of sodium oxybate in each tablet with a delivery profile similar to that of the liquid form. The tablets are bioequivalent to the liquid form.
Owner:JAZZ PHARMA INC
Tacrolimus for improved treatment of transplant patients
ActiveUS20100105717A1Improve bioavailabilityReduce riskBiocideOrganic chemistryTherapeutic effectIn vivo
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARM INC
Immediate release dosage forms containing solid drug dispersions
ActiveUS9211261B2High strengthIncreased durabilityPowder deliveryDrug compositionsWater solubleUltimate tensile strength
High loading immediate release dosage forms containing at least 30 wt % of a solid drug dispersion, at least 5 wt % of a disintegrant and a porosigen are disclosed that exhibit excellent strength and aqueous solubility.
Owner:PFIZER PROD INC +1
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition.
Owner:ELAN PHRMA INT LTD
Immediate release dosage forms containing solid drug disbursions
ActiveUS20080317851A1Quick releaseHigh strengthPowder deliveryDrug compositionsWater solubleUltimate tensile strength
High loading immediate release dosage forms containing at least 30 wt % of a solid drug dispersion, at least 5 wt % of a disintegrant and a porosigen are disclosed that exhibit excellent strength and aqueous solubility.
Owner:PFIZER PROD INC +1
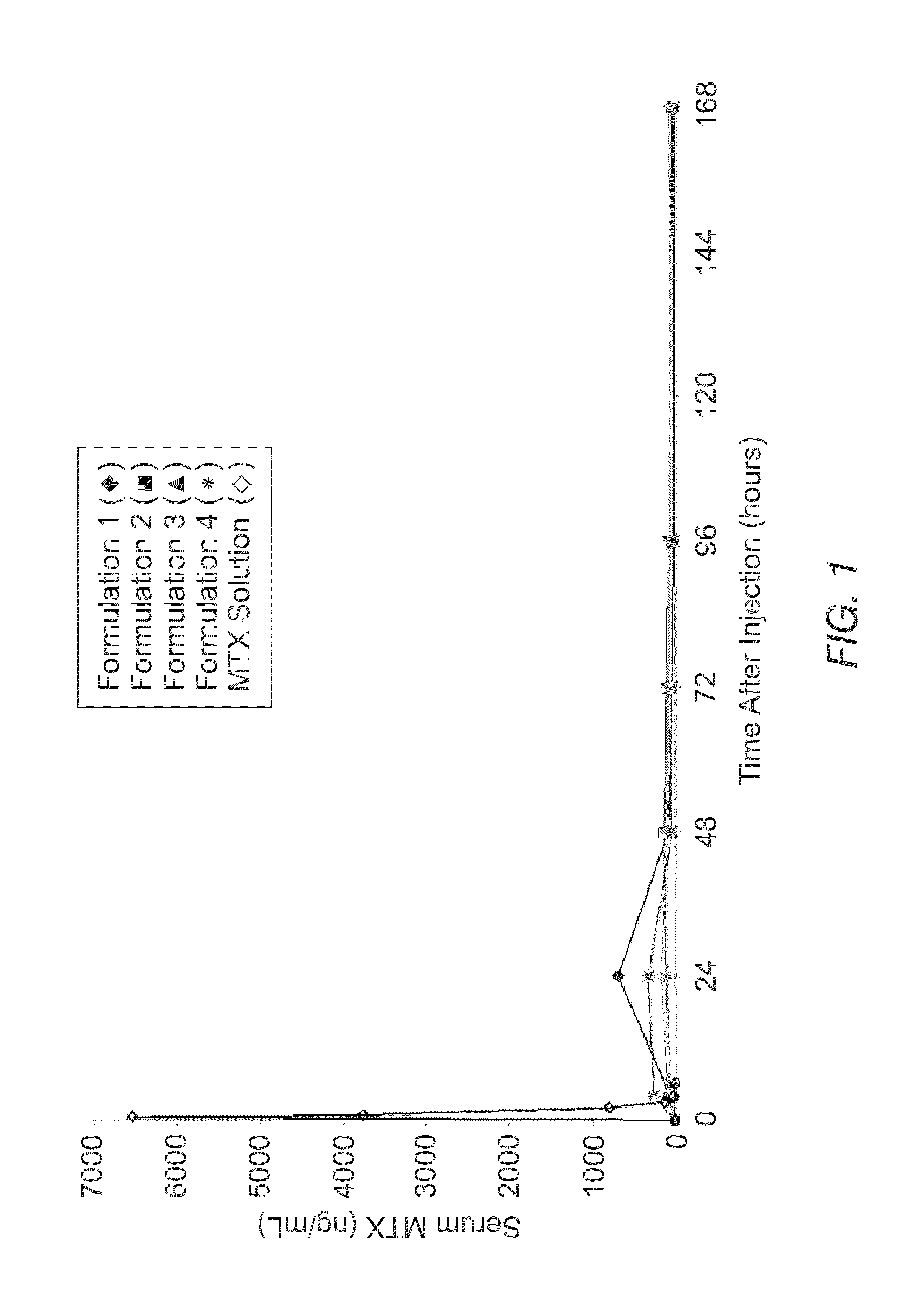
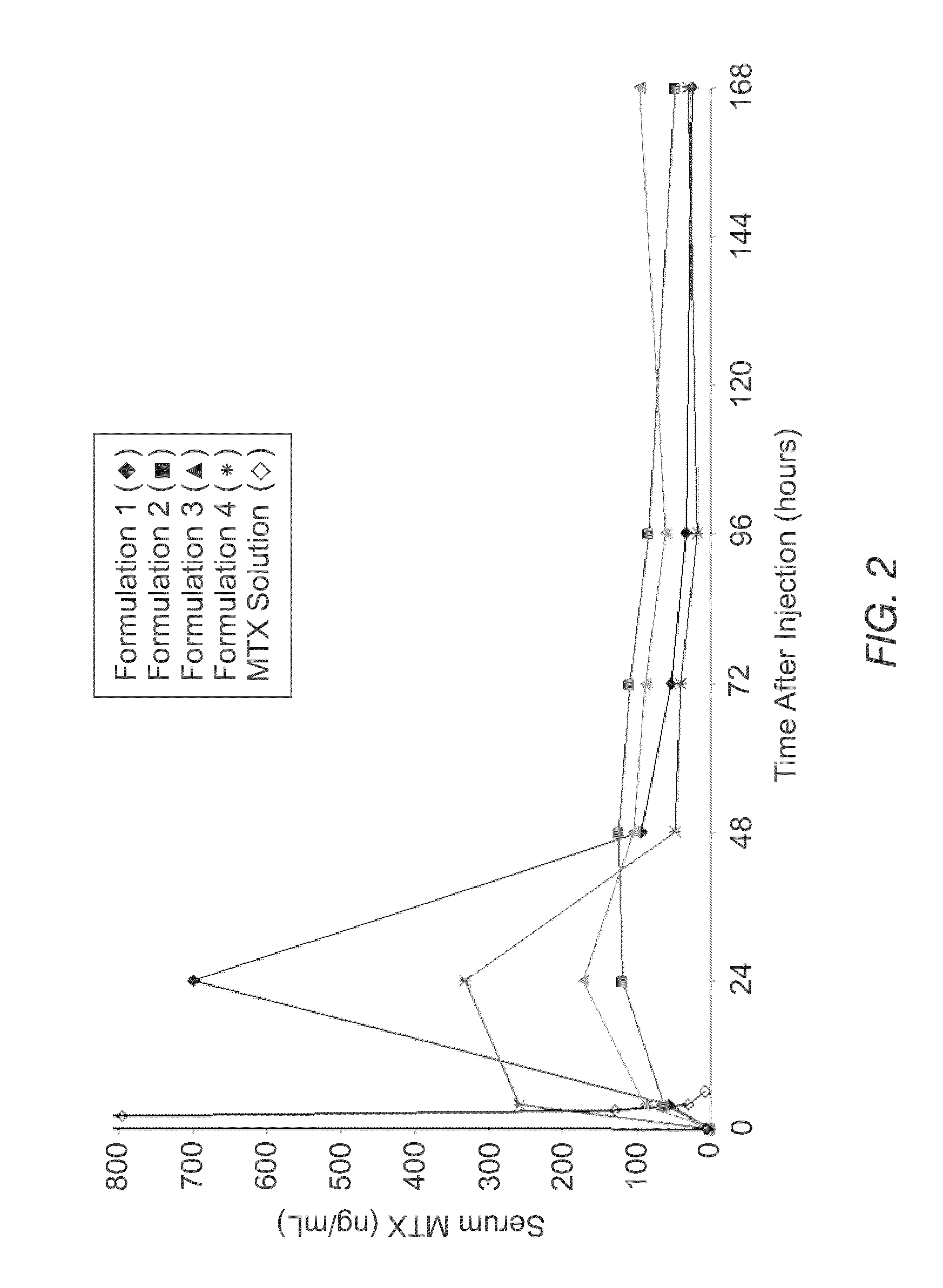
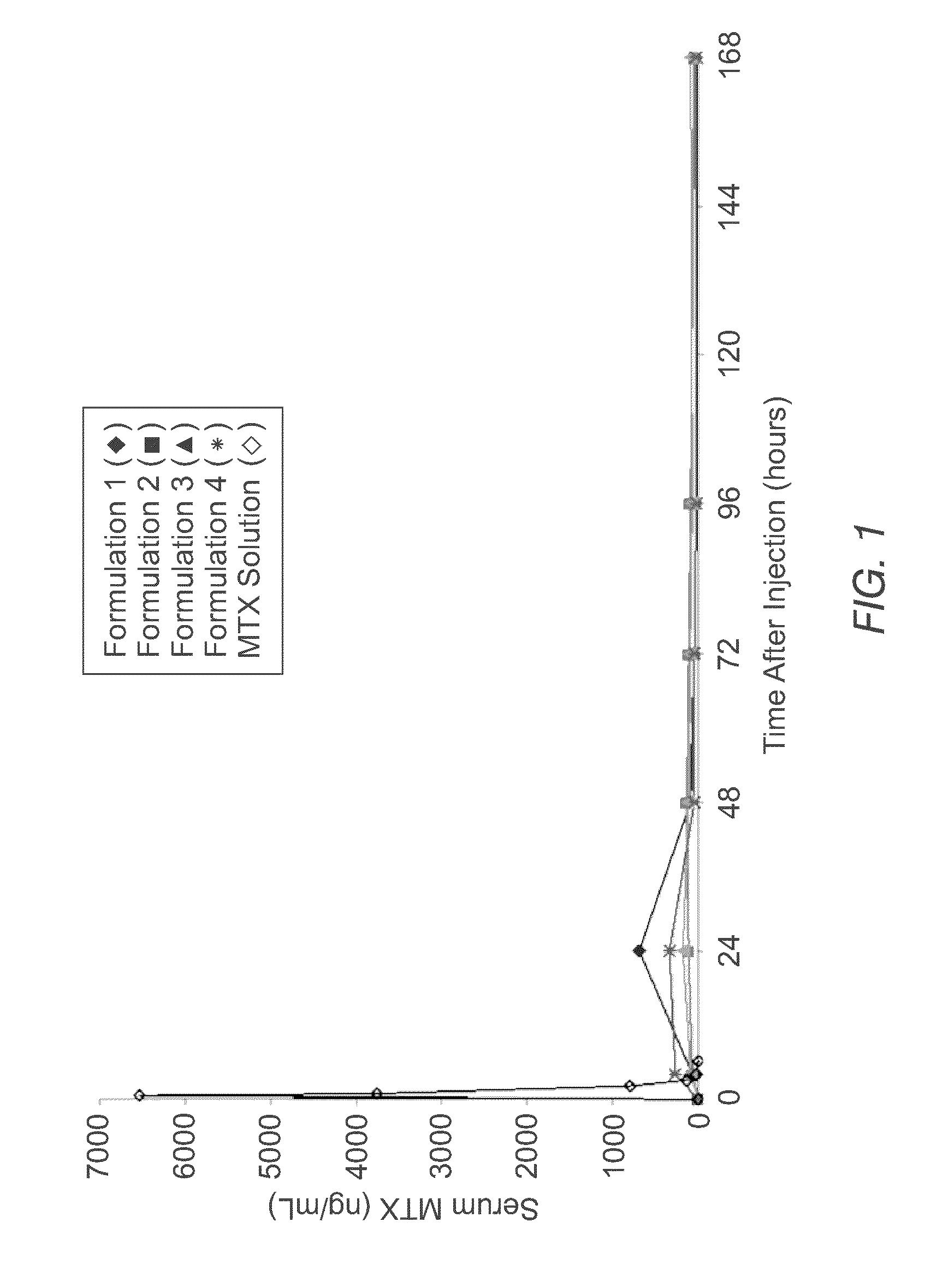
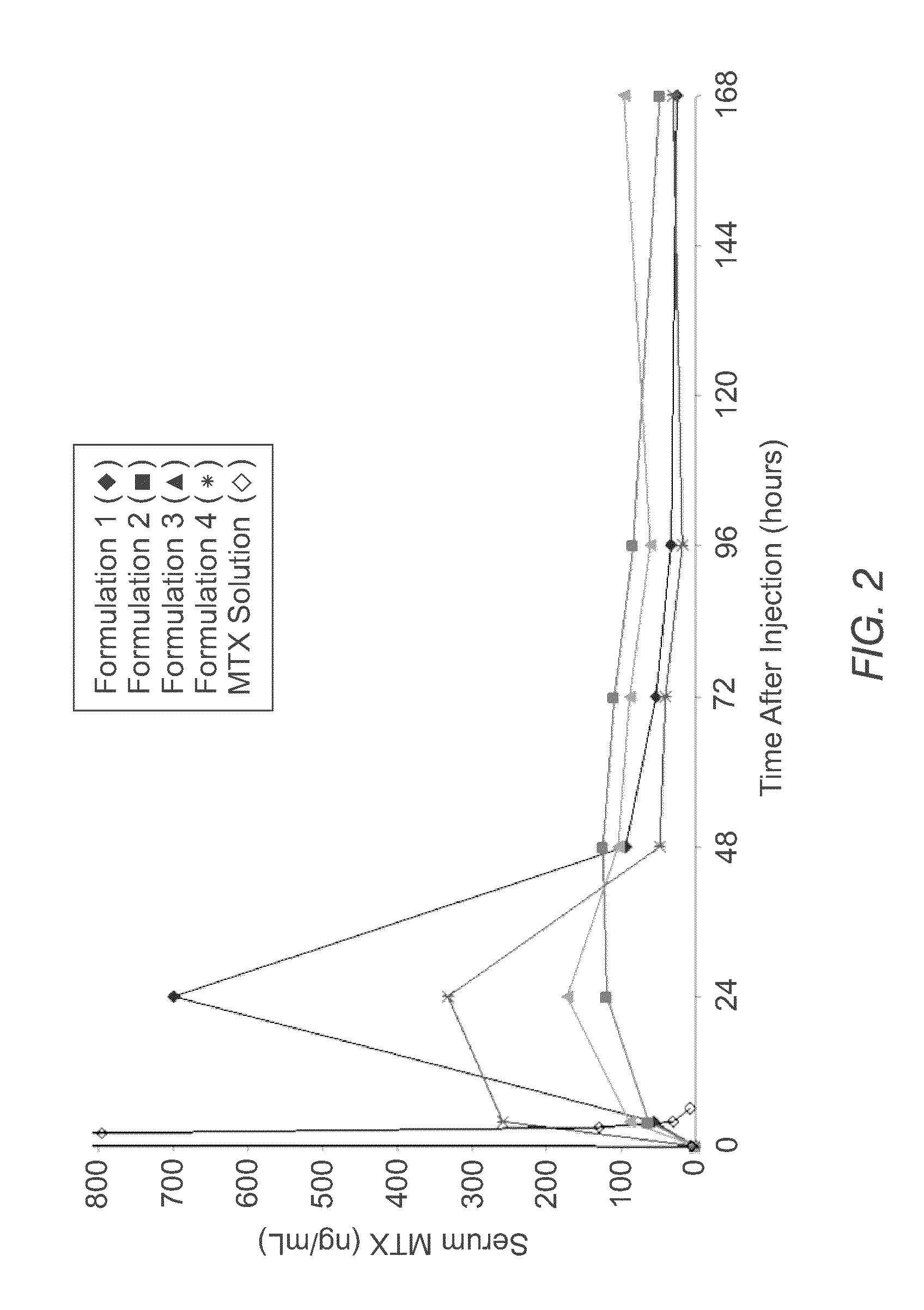
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an Anti-cancer agent
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
Methods and dosage forms for controlled delivery of paliperidone and risperidone
InactiveUS20050232995A1Eliminate side effectsImprove development of toleranceOrganic active ingredientsBiocideDosing regimenRegimen
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Tacrolimus For Improved Treatment Of Transplant Patients
InactiveUS20110275659A1Reduce riskAffect bioavailabilityBiocidePharmaceutical non-active ingredientsIn vivoBioavailability
An extended release oral dosage form comprising as active substance tacrolimus or a pharmaceutically active analogue thereof for a once daily immunosuppressive treatment of a patient in need thereof, preferable a kidney or liver transplant patient. The dosage form releases the active substance over an extended period of time. It also provides improved pharmacokinetic parameters due to an extended and constant in vivo release including substantial decreased peak concentrations, despite increased bioavailability, substantial extended times for maximal concentration, and higher minimal concentrations when compared with conventional immediate release dosage forms and a recent modified release tacrolimus dosage form.
Owner:VELOXIS PHARMA
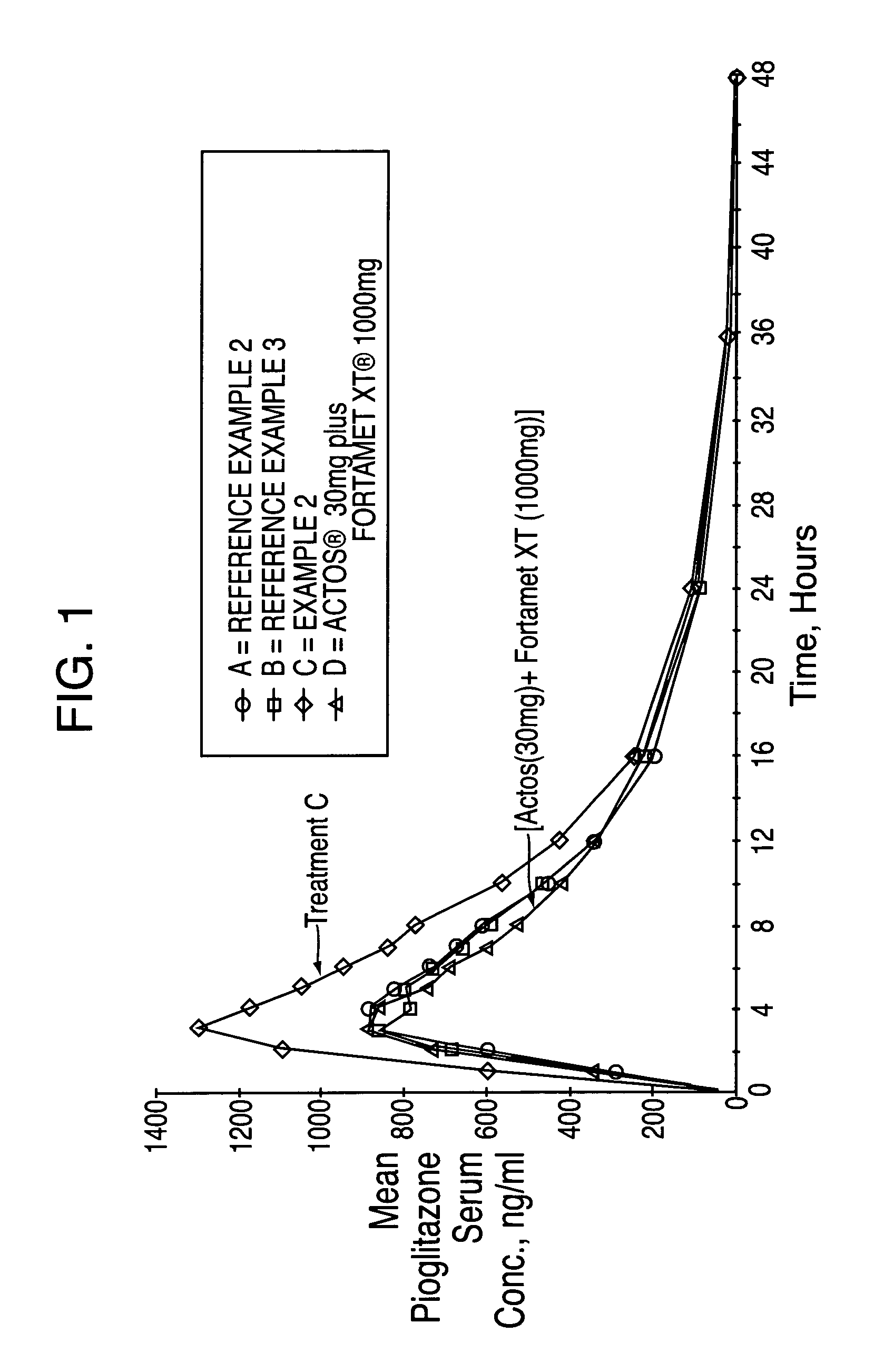
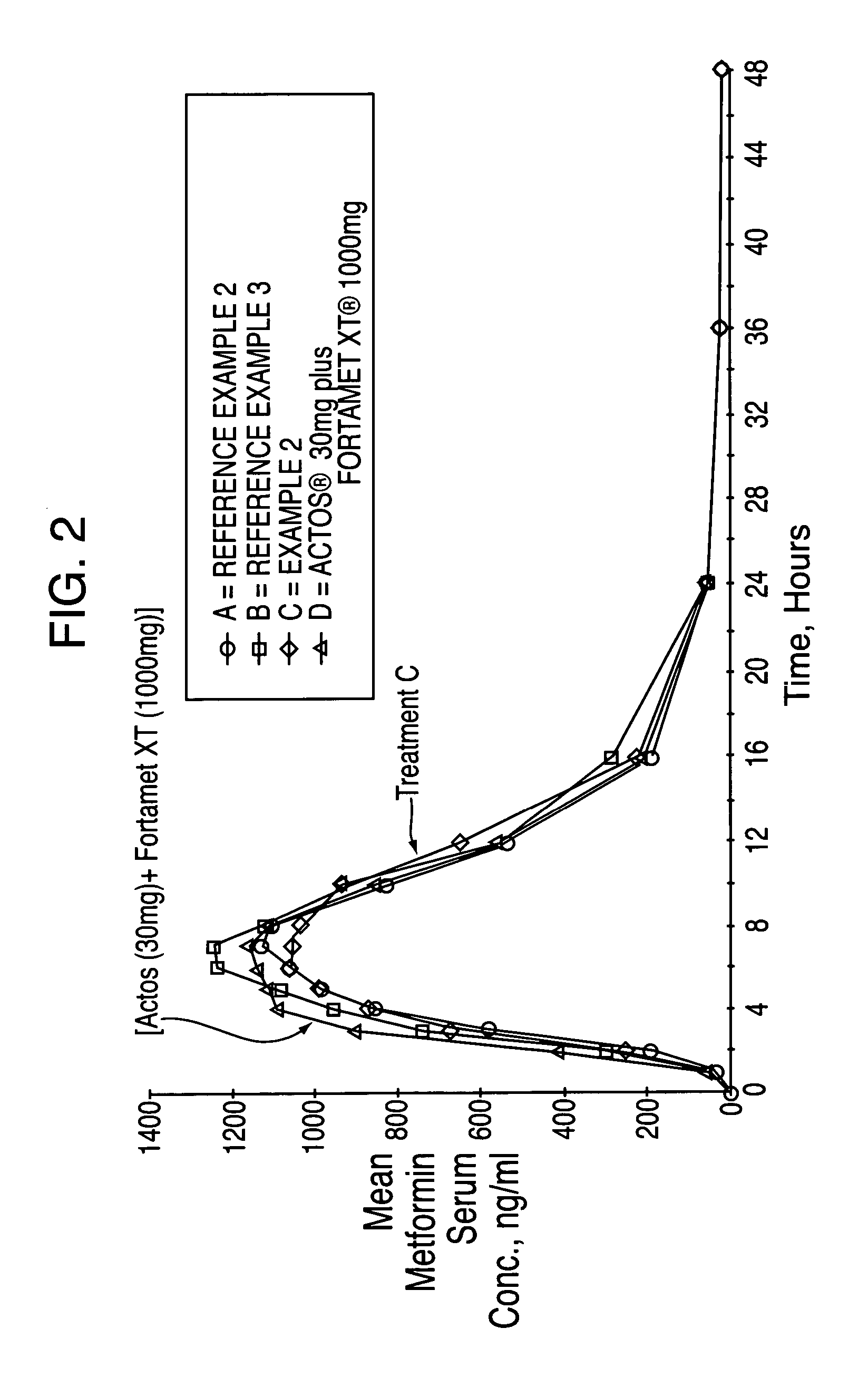
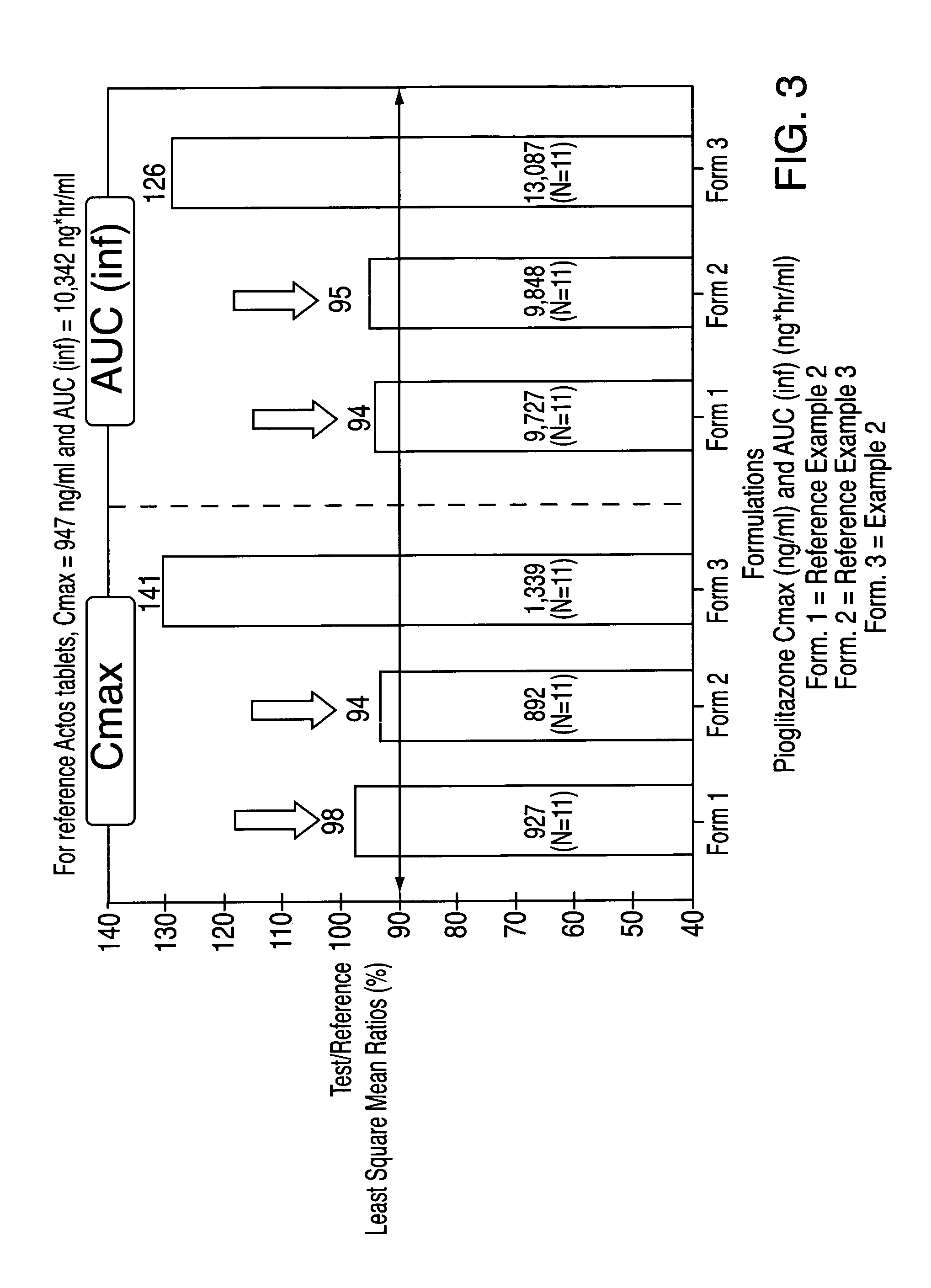
Novel pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative
ActiveUS20050249809A1Improve bioavailabilityIncrease and high bioavailabilityOrganic active ingredientsBiocideControlled releaseThiazolidinedione
A pharmaceutical dosage form comprising a controlled release component comprising an antihyperglycemic drug in combination with a second component comprising a thiazolidinedione derivative and a disintegrating agent is herein disclosed and described. The dosage formulation exhibits a significant increase in bioavailability of the thiazolidinedione derivative component compared to conventional immediate release dosage forms containing only a thiazolidinedione derivative.
Owner:TAKEDA PHARMA CO LTD +1
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an anti-cancer agent
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
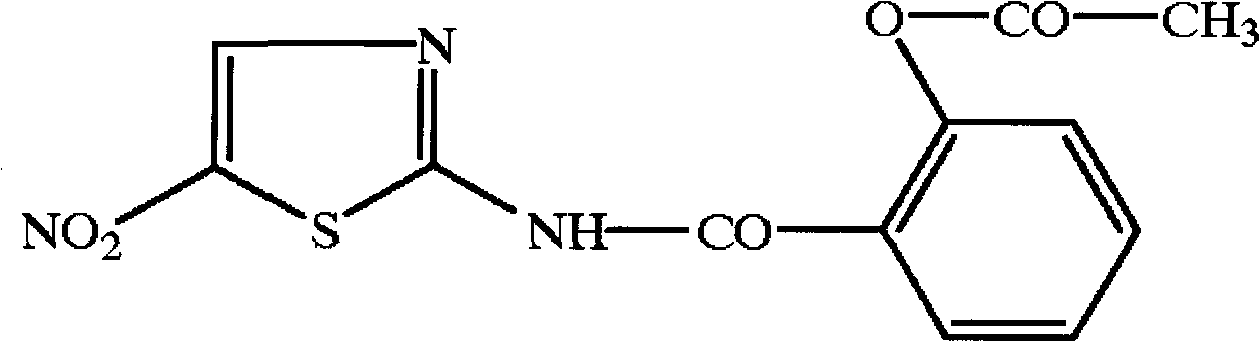
Controlled release pharmaceutical formulations of nitazoxanide
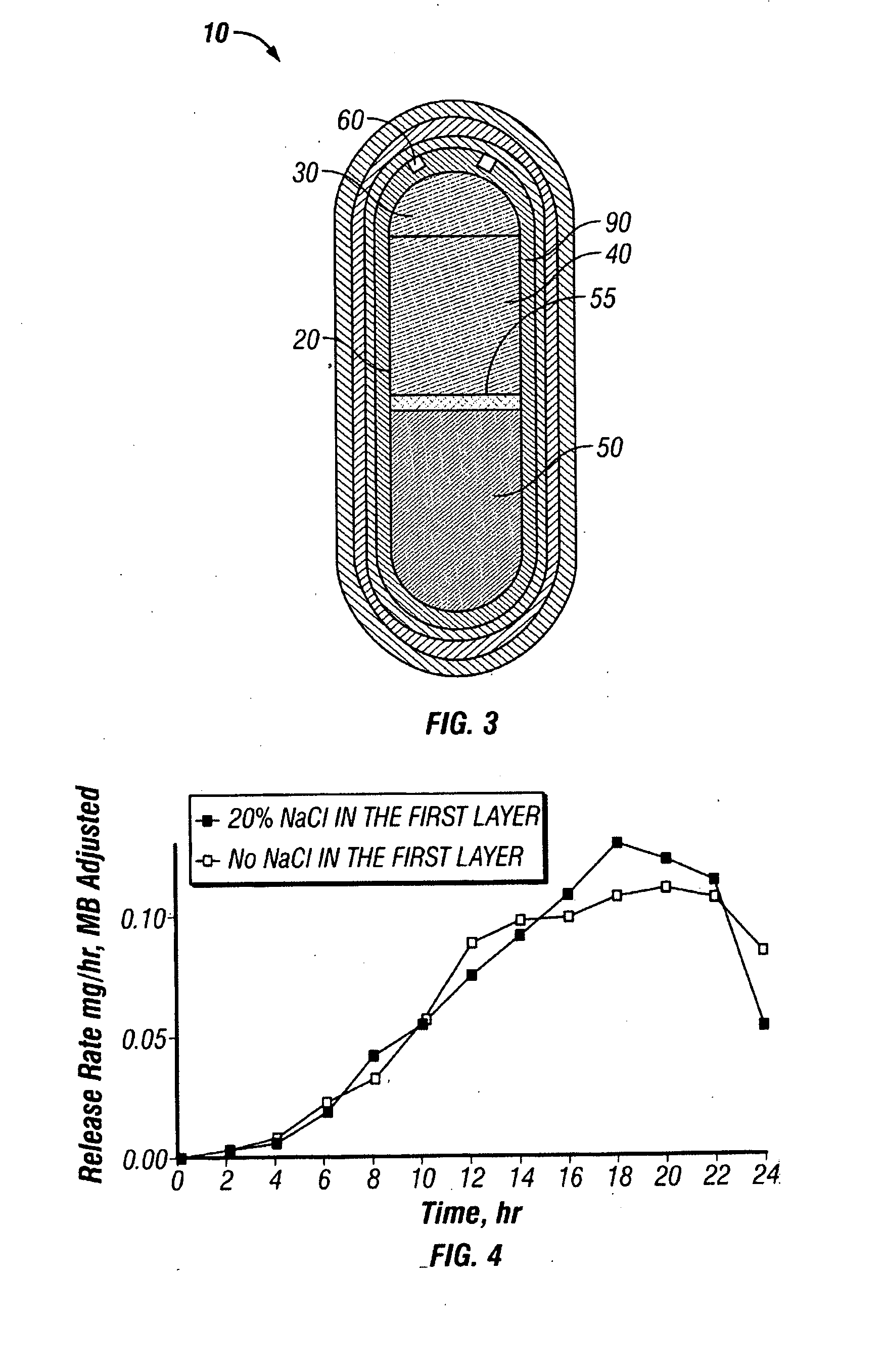
Solid dosage formulations of nitazoxanide or a nitazoxanide analogue are provided that comprise a controlled release portion and an immediate release portion. The pharmaceutical composition is typically in the form of a bilayer solid oral dosage form comprising (a) a first layer comprising a first quantity of nitazoxanide or analogue thereof in a controlled release formulation; and (b) a second layer comprising a second quantity of nitazoxanide or analogue thereof in an immediate release formulation. Method of using the formulations in the treatment of hepatitis C are also provided.
Owner:ROMARK LAB L C
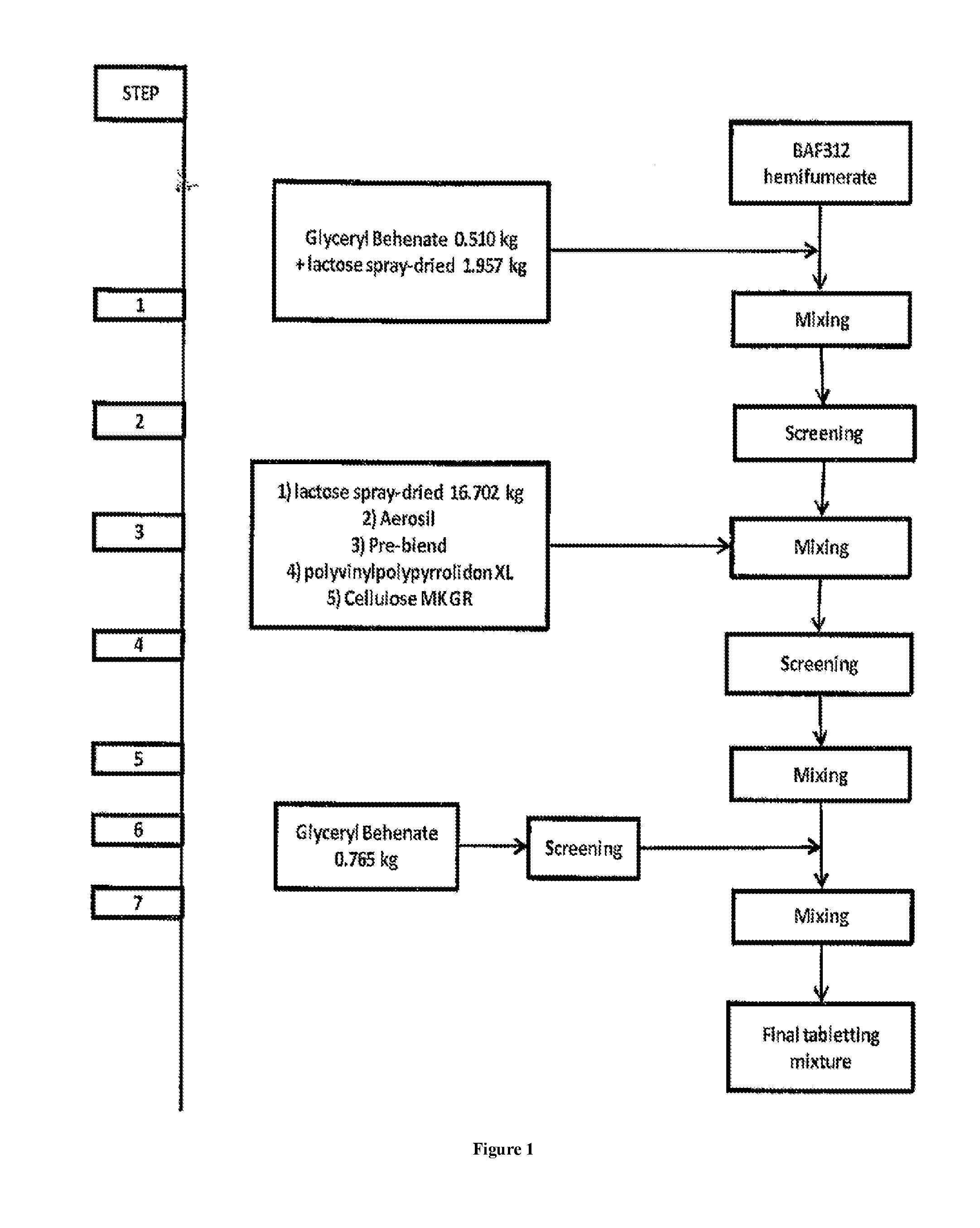
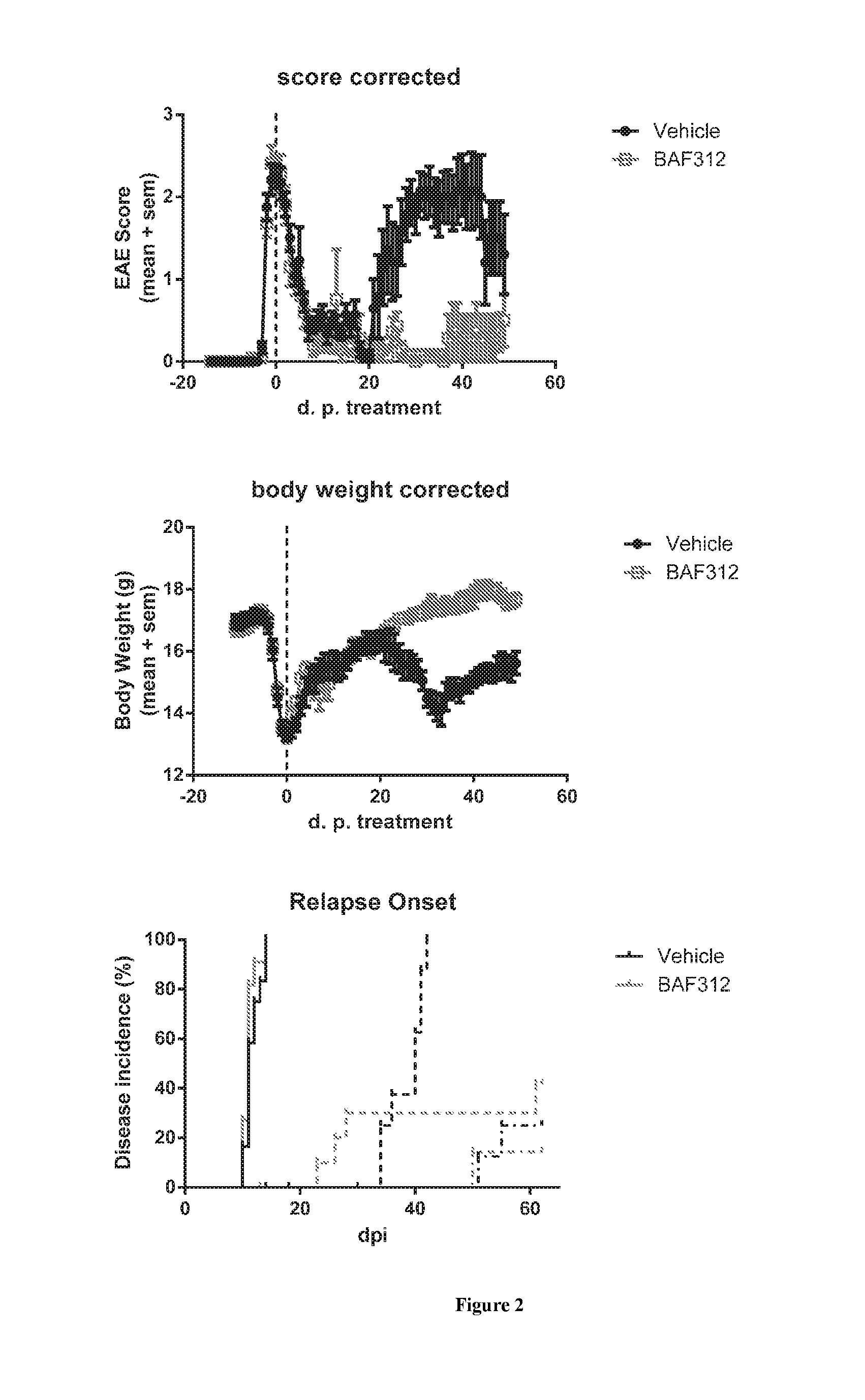
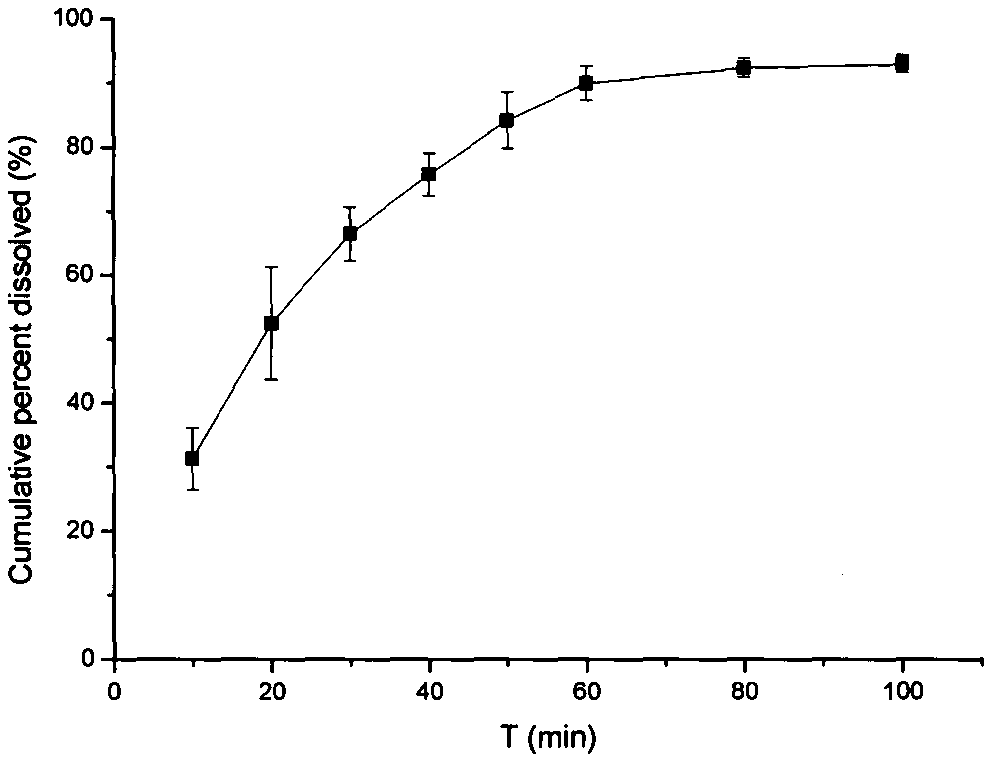
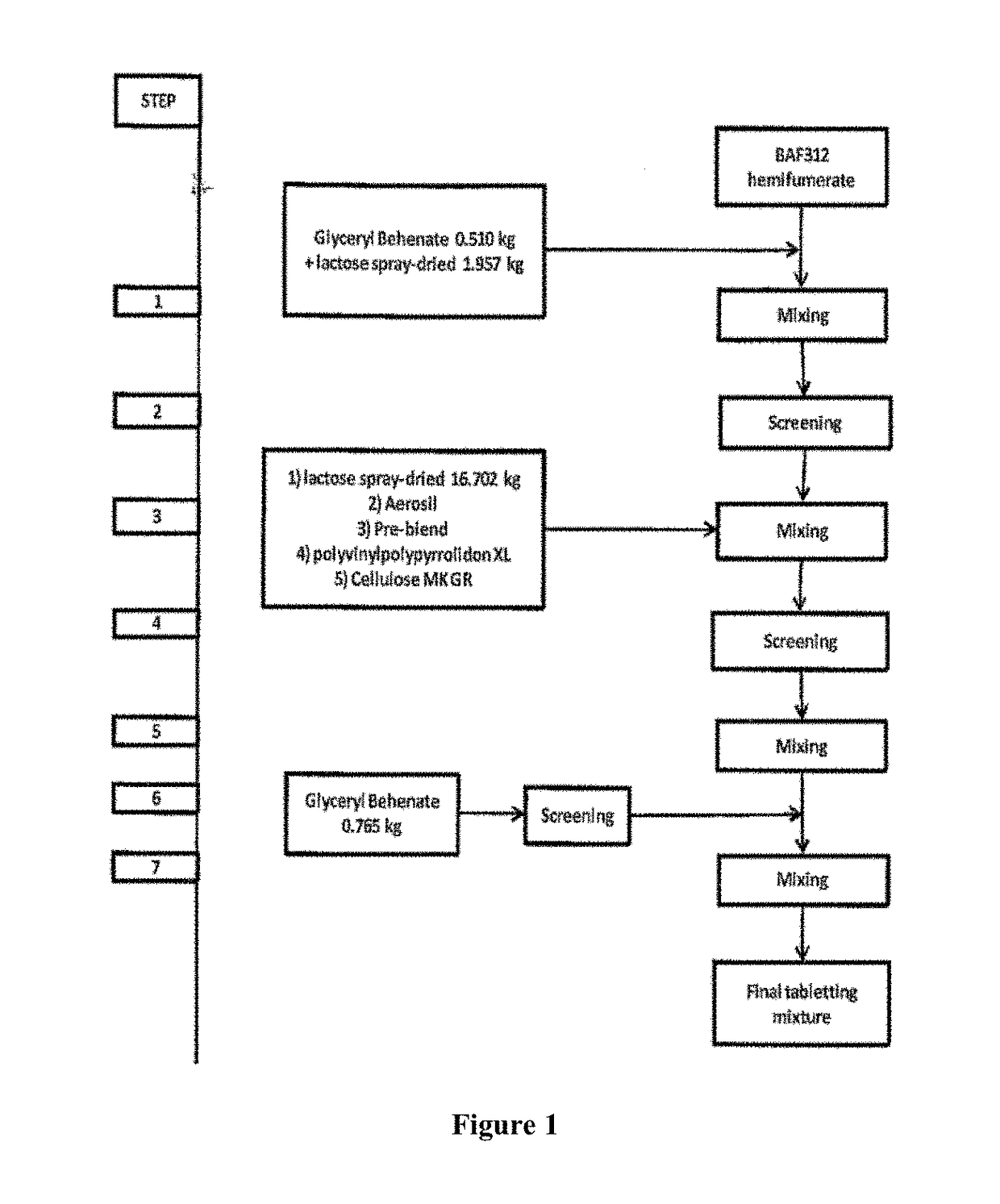
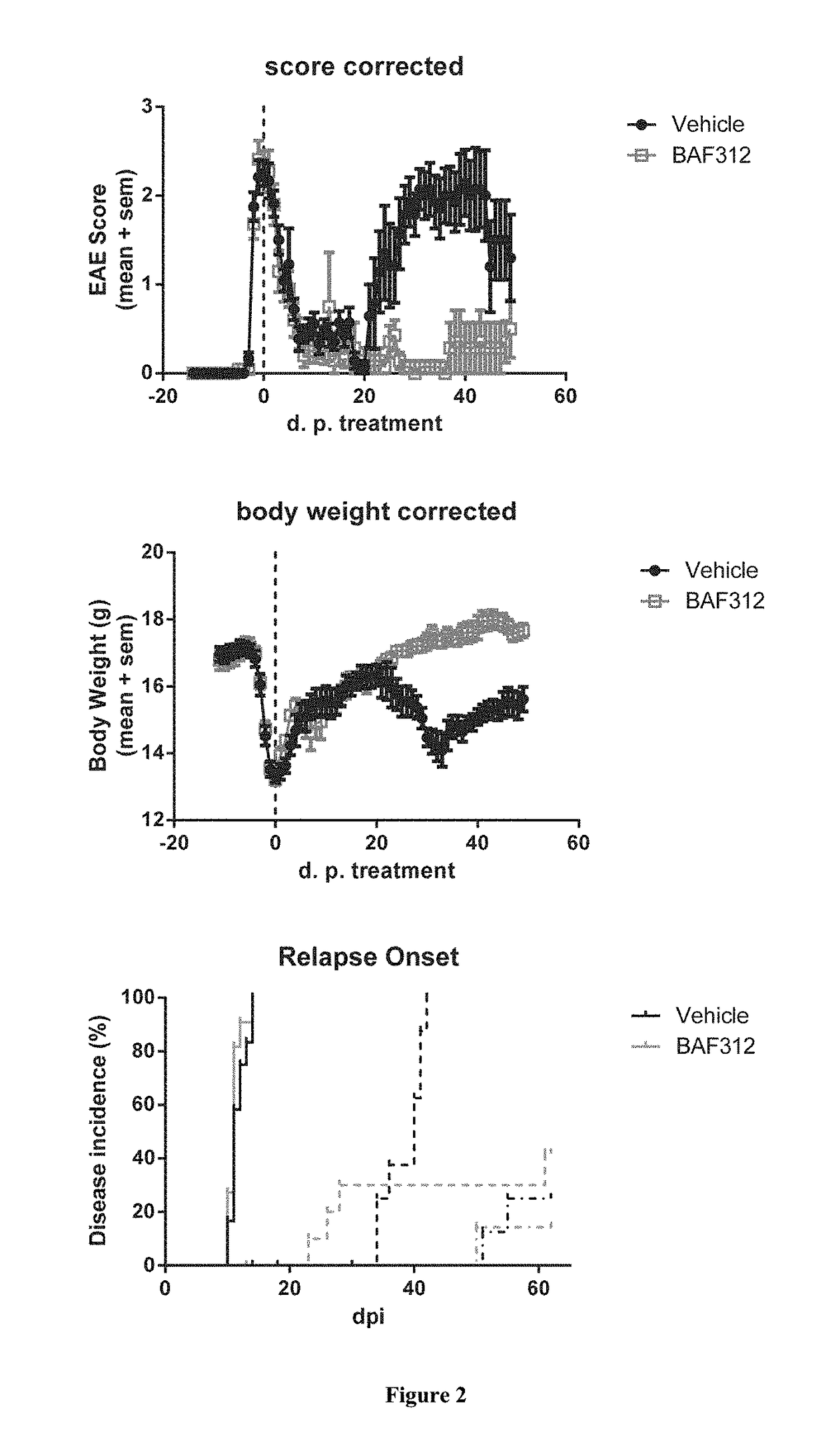
Sip modulator immediate release dosage regimen
The present invention relates to siponimod (BAF312) for use in the treatment of an autoimmune disease, wherein an immediate release dosage form is administered once daily to a patient as maintenance regimen and wherein the patient has experienced a specific titration regimen with siponimod beforehand.
Owner:NOVARTIS AG
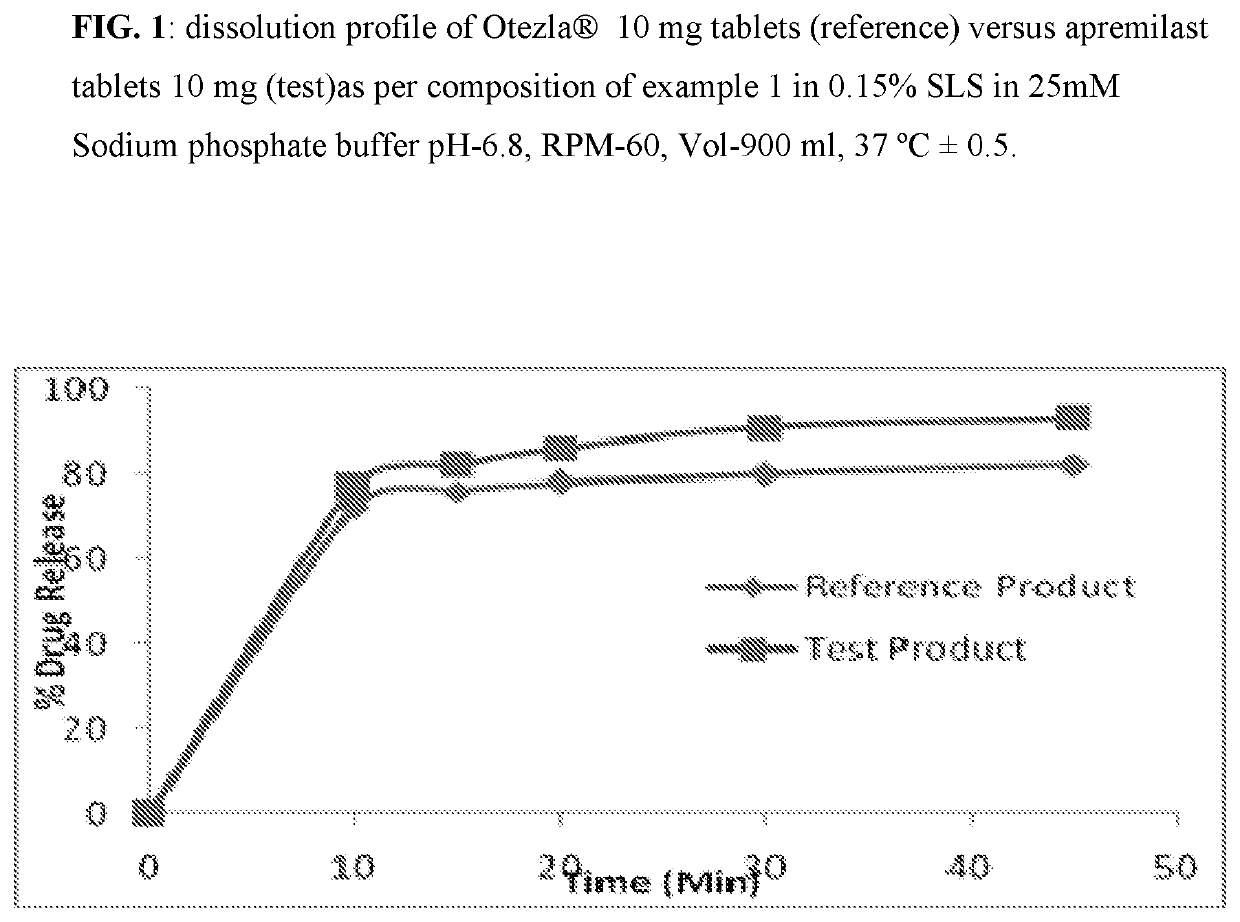
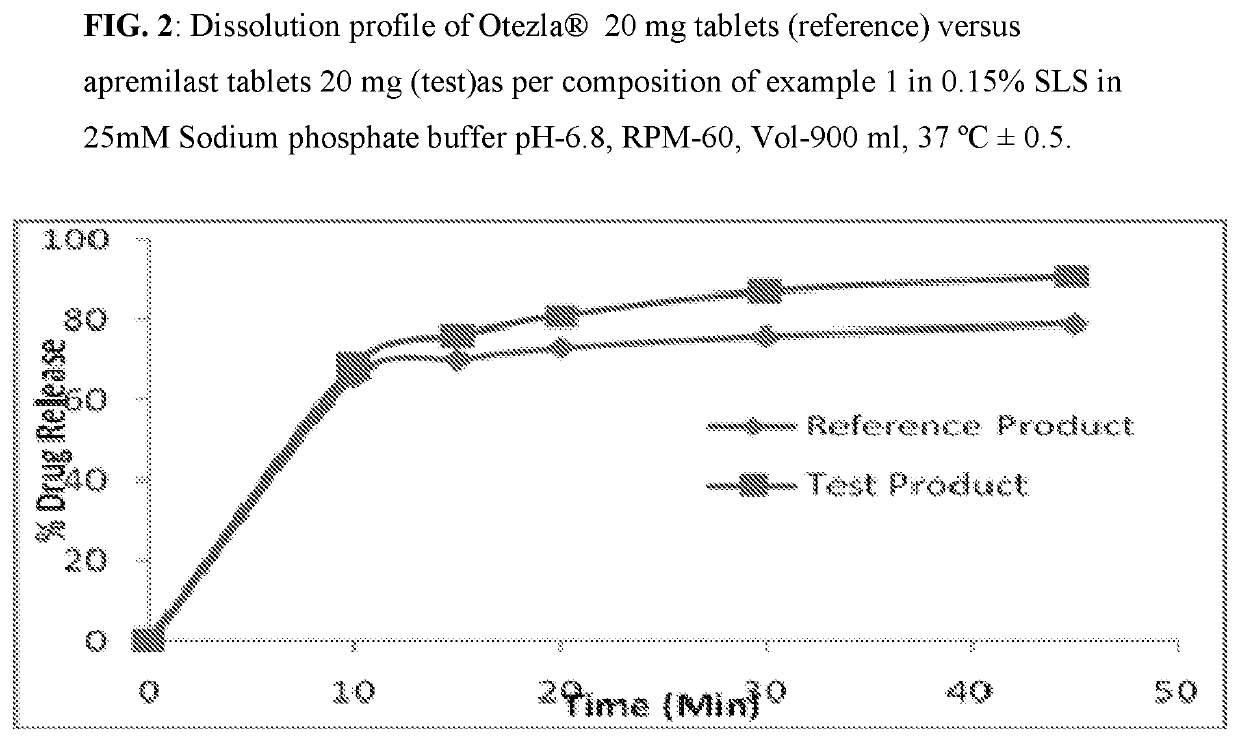
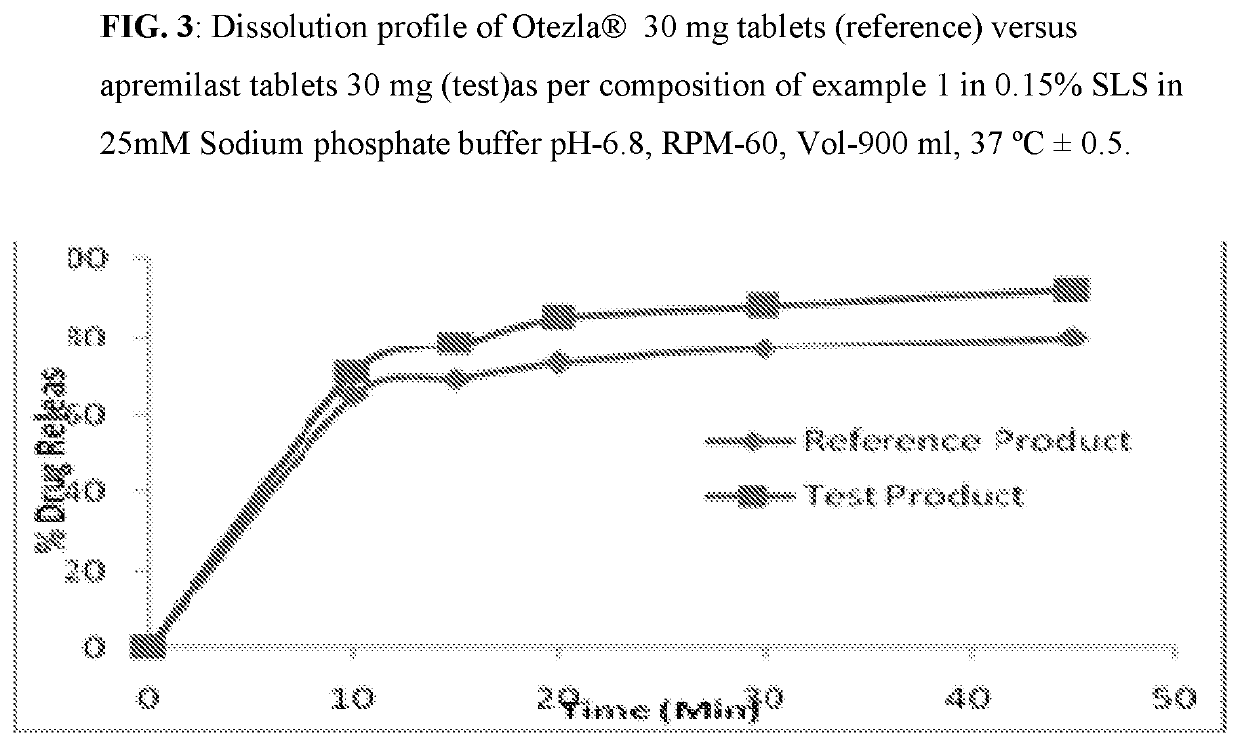
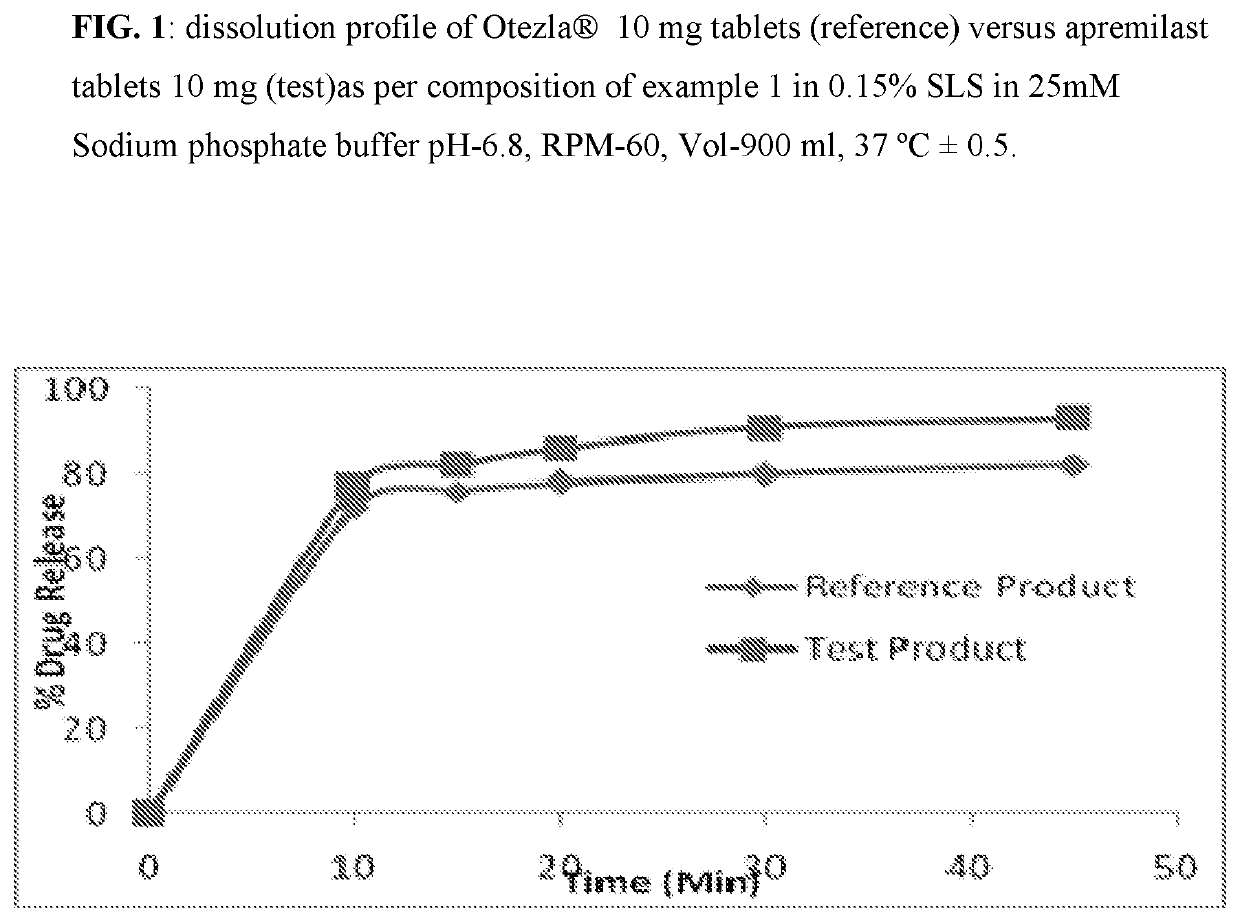
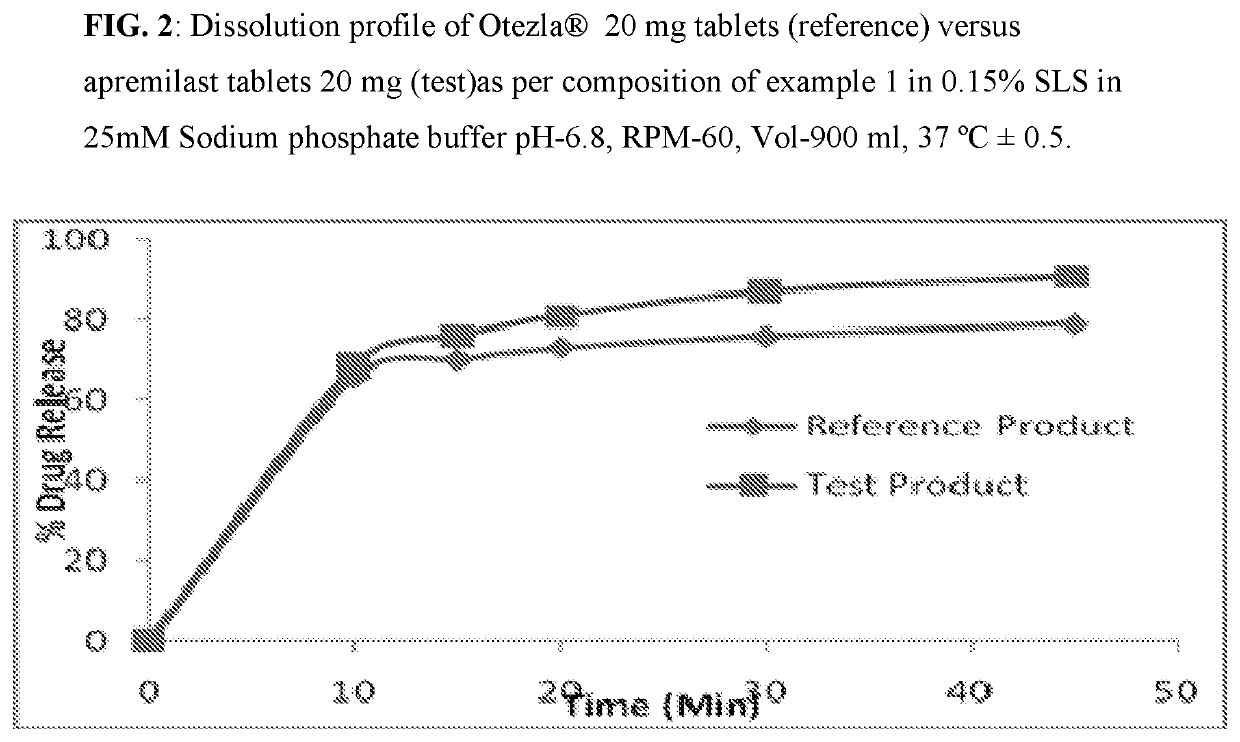
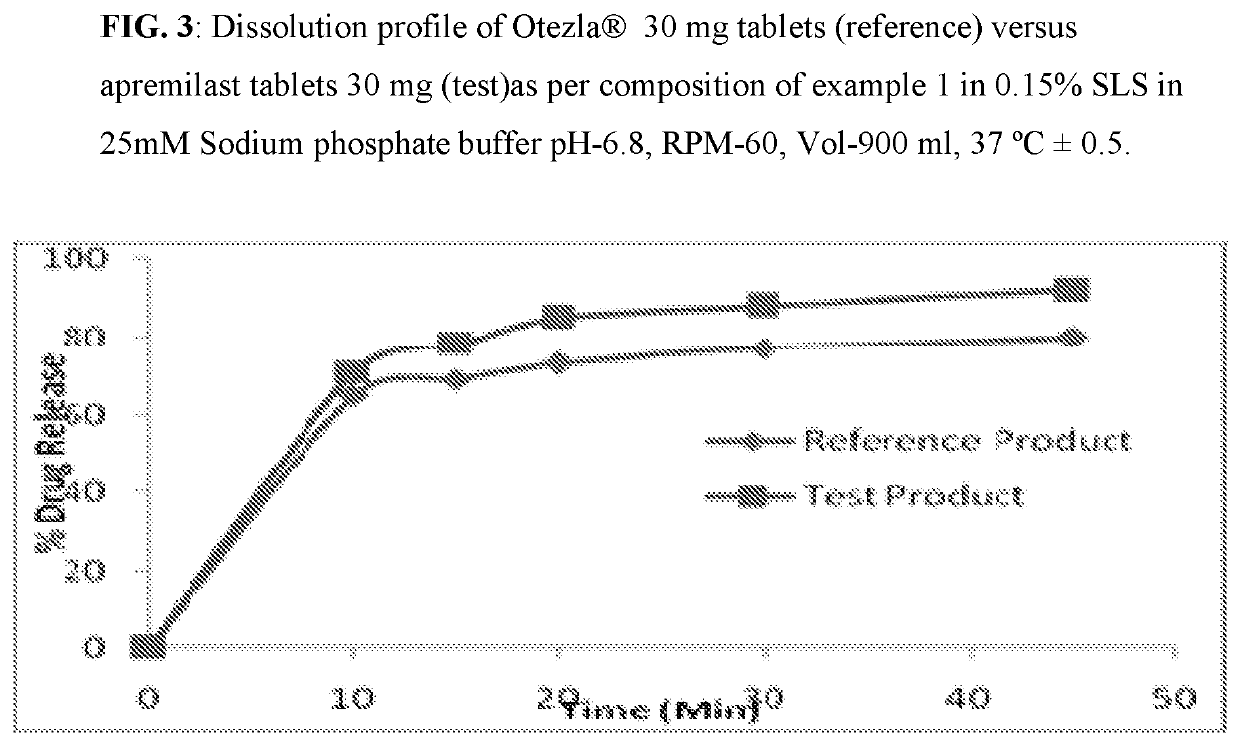
Pharmaceutical compositions of apremilast
The present invention relates to a disintegrant free pharmaceutical composition comprising apremilast or pharmaceutically acceptable salts thereof and one or more pharmaceutically acceptable excipients. Particularly the present invention relates to disintegrant free immediate release dosage form comprising apremilast or pharmaceutically acceptable salts thereof and one or more pharmaceutically acceptable excipients. It further relates to process of preparing such composition and its use in psoriatic arthritis and psoriasis.
Owner:UNICHEM LAB LTD
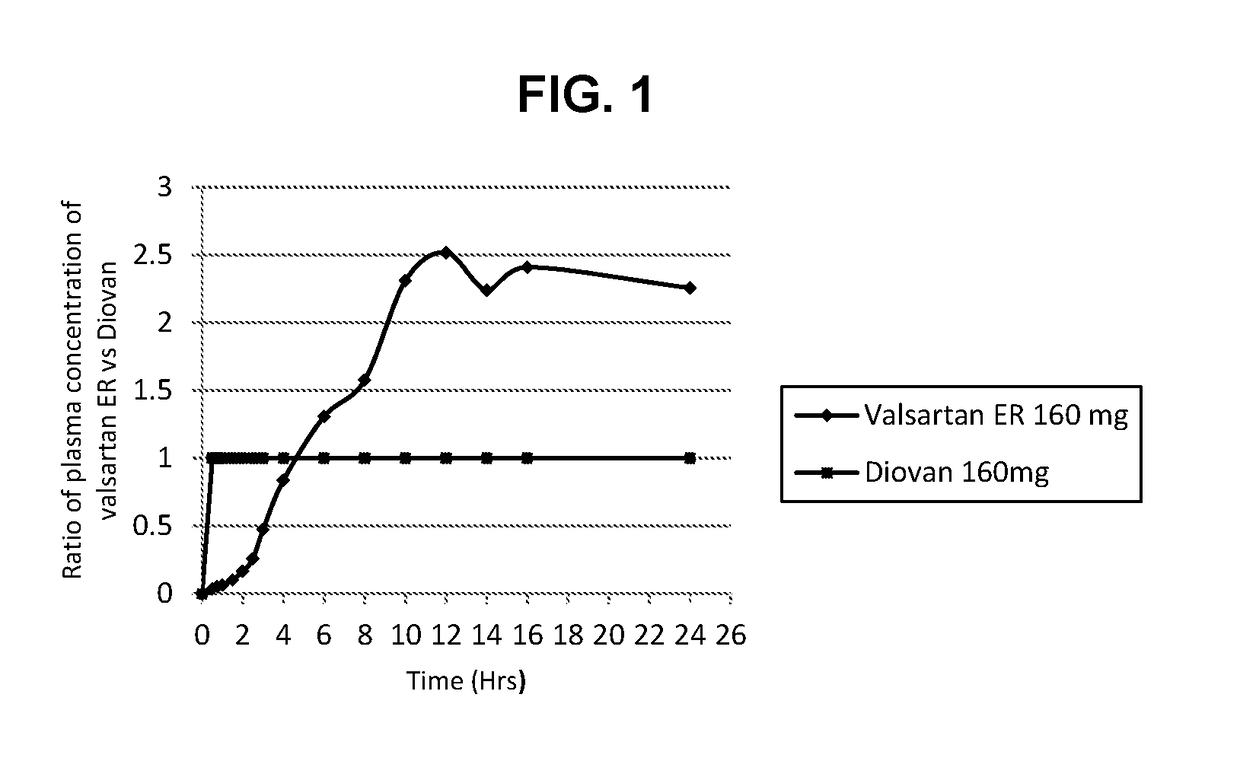
Methods and composition for treatment of cardiovascular conditions
The present invention relates to methods for treatment of cardiovascular conditions selected from high blood pressure, heart failure, or heart attack. The present invention further relates to a method of treating cardiovascular conditions comprising orally administering once a day to a human subject in need thereof the compound valsartan in an extended release dosage form, wherein the ratio of mean plasma concentration of valsartan provided by the extended release dosage form to the mean plasma concentration of valsartan provided by an immediate release dosage form of valsartan over 8 hour to 24 hour period after administration is greater than 1 in a single dose human pharmacokinetic study.
Owner:EZRA PHARMA LLC +1
Pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative
A pharmaceutical dosage form comprising a controlled release component comprising an antihyperglycemic drug in combination with a second component comprising a thiazolidinedione derivative and a disintegrating agent is herein disclosed and described. The dosage formulation exhibits a significant increase in bioavailability of the thiazolidinedione derivative component compared to conventional immediate release dosage forms containing only a thiazolidinedione derivative.
Owner:TAKEDA PHARMA CO LTD +1
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an Anti-cancer agent
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
Immediate release pharmaceutical composition of iron chelating agents
ActiveUS10888519B2High drug loadingImprovement ingredientsOrganic active ingredientsBlood disorderPharmaceutical drugDeferasirox
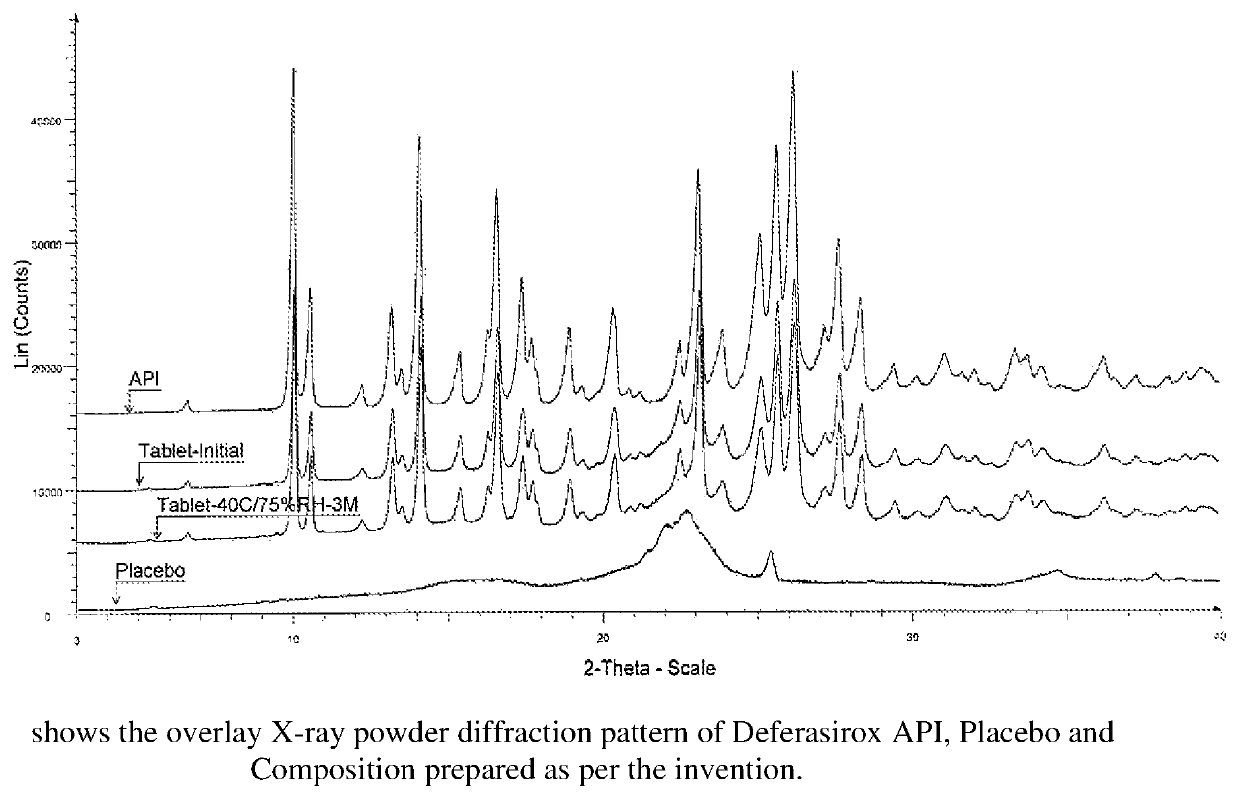
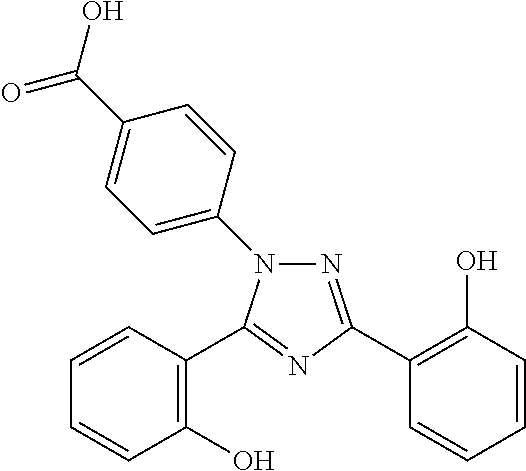
The present invention relates to a stable, immediate release solid oral pharmaceutical compositions comprising iron chelating agents like Deferasirox and at least one pharmaceutical acceptable excipient wherein the composition is free of glidant. Prior art discloses various technical challenges and suggest restrictive and complex solutions for the development of immediate release dosage forms of Deferasirox such as utilizing a large number of excipients or non-conventional formulation techniques. The glidant free immediate release solid oral pharmaceutical composition of Deferasirox, prepared as per present invention exhibited desirable technical attributes like pharmaceutical stability, flow properties and comparable dissolution, bioequivalence against reference listed drug.
Owner:JUBILANT GENERICS
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an Anti-cancer agent
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
Controlled release pharmaceutical formulations of nitazoxanide
InactiveCN107260695AOrganic active ingredientsPeptide/protein ingredientsPharmaceutical formulationControlled-Release Formulations
Solid dosage formulations of nitazoxanide or a nitazoxanide analogue are provided that comprise a controlled release portion and an immediate release portion. The pharmaceutical composition is typically in the form of a bilayer solid oral dosage form comprising (a) a first layer comprising a first quantity of nitazoxanide or analogue thereof in a controlled release formulation; and (b) a second layer comprising a second quantity of nitazoxanide or analogue thereof in an immediate release formulation. Method of using the formulations in the treatment of hepatitis C are also provided.
Owner:ROMARK LAB L C
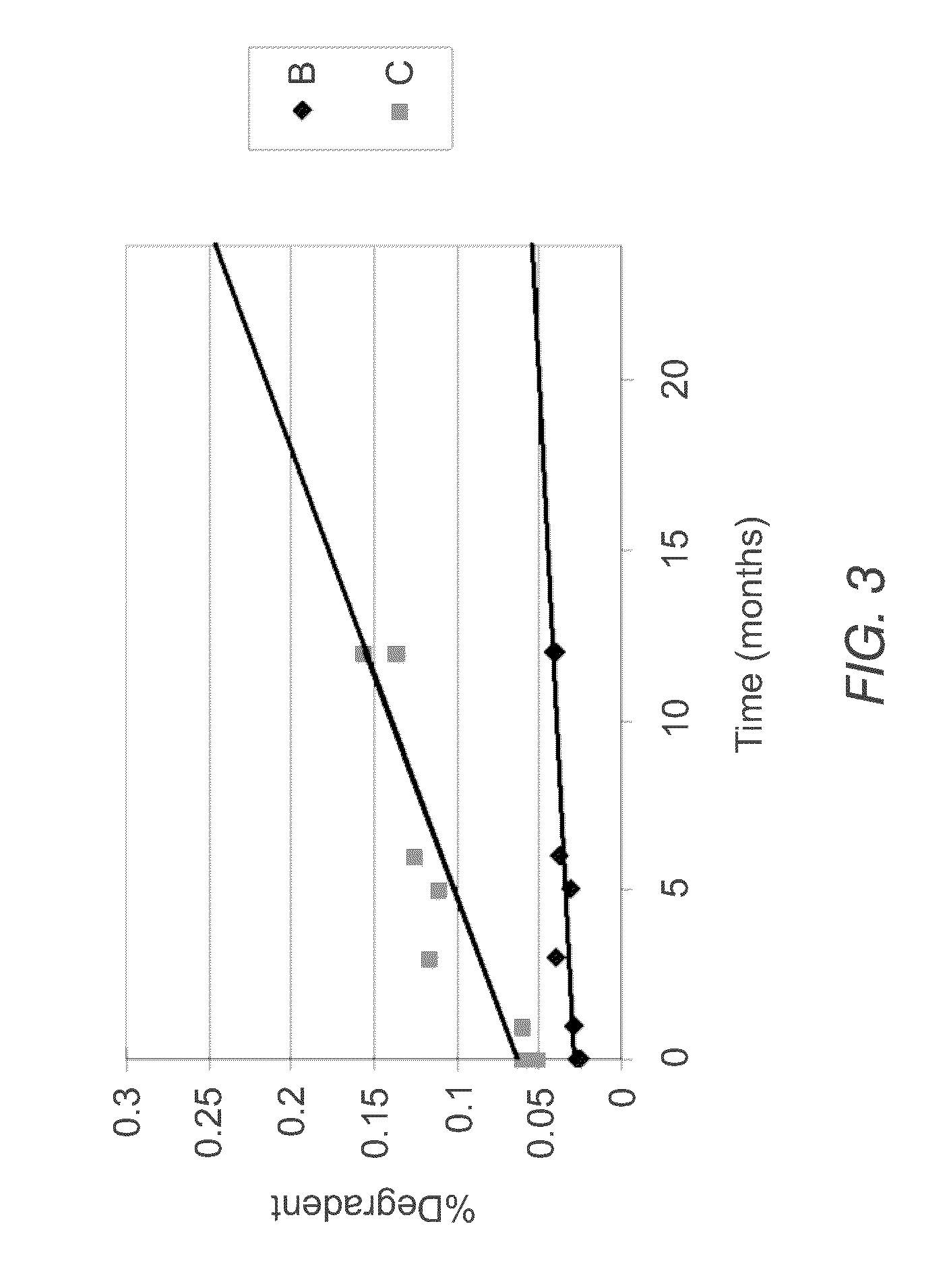
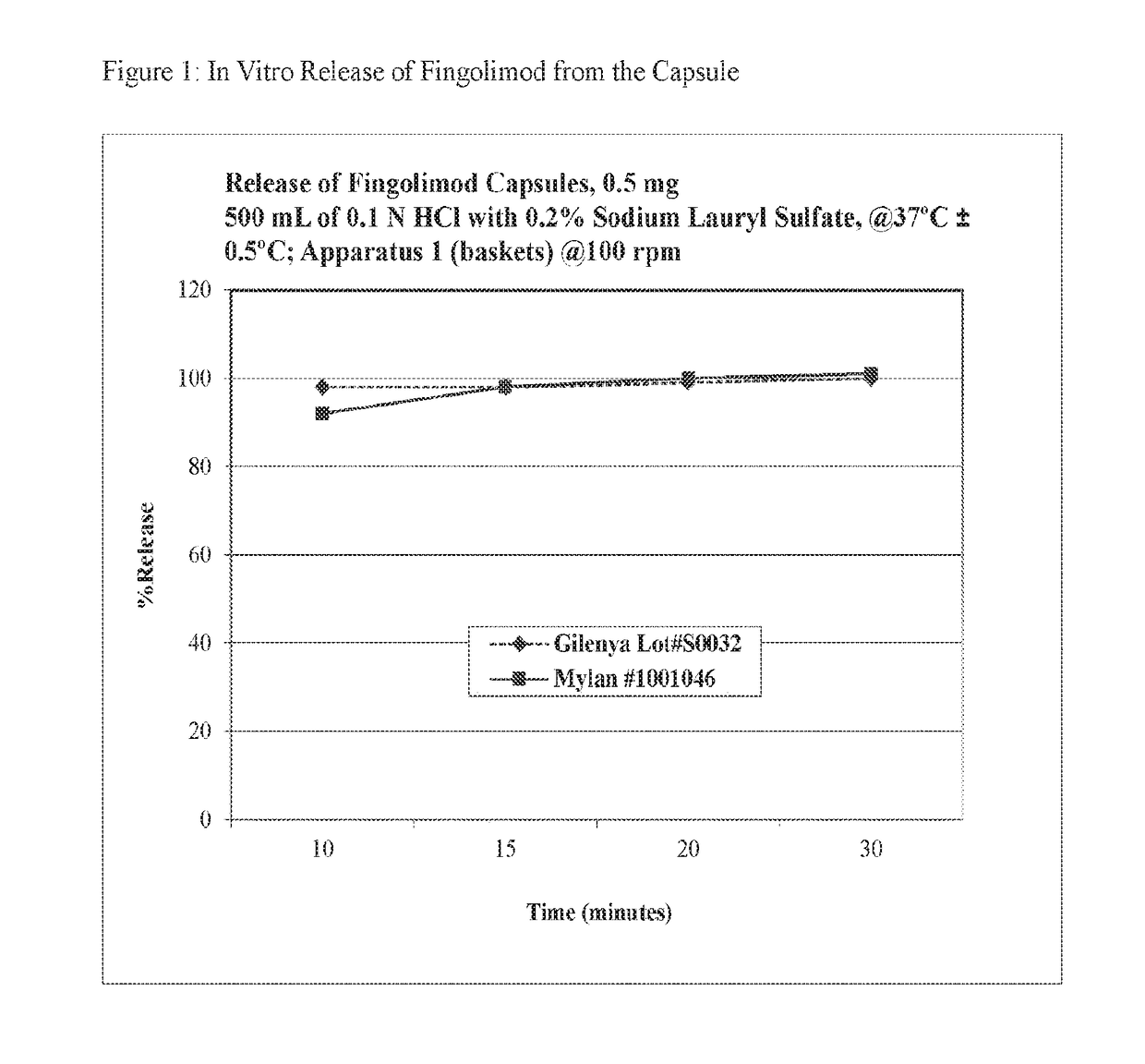
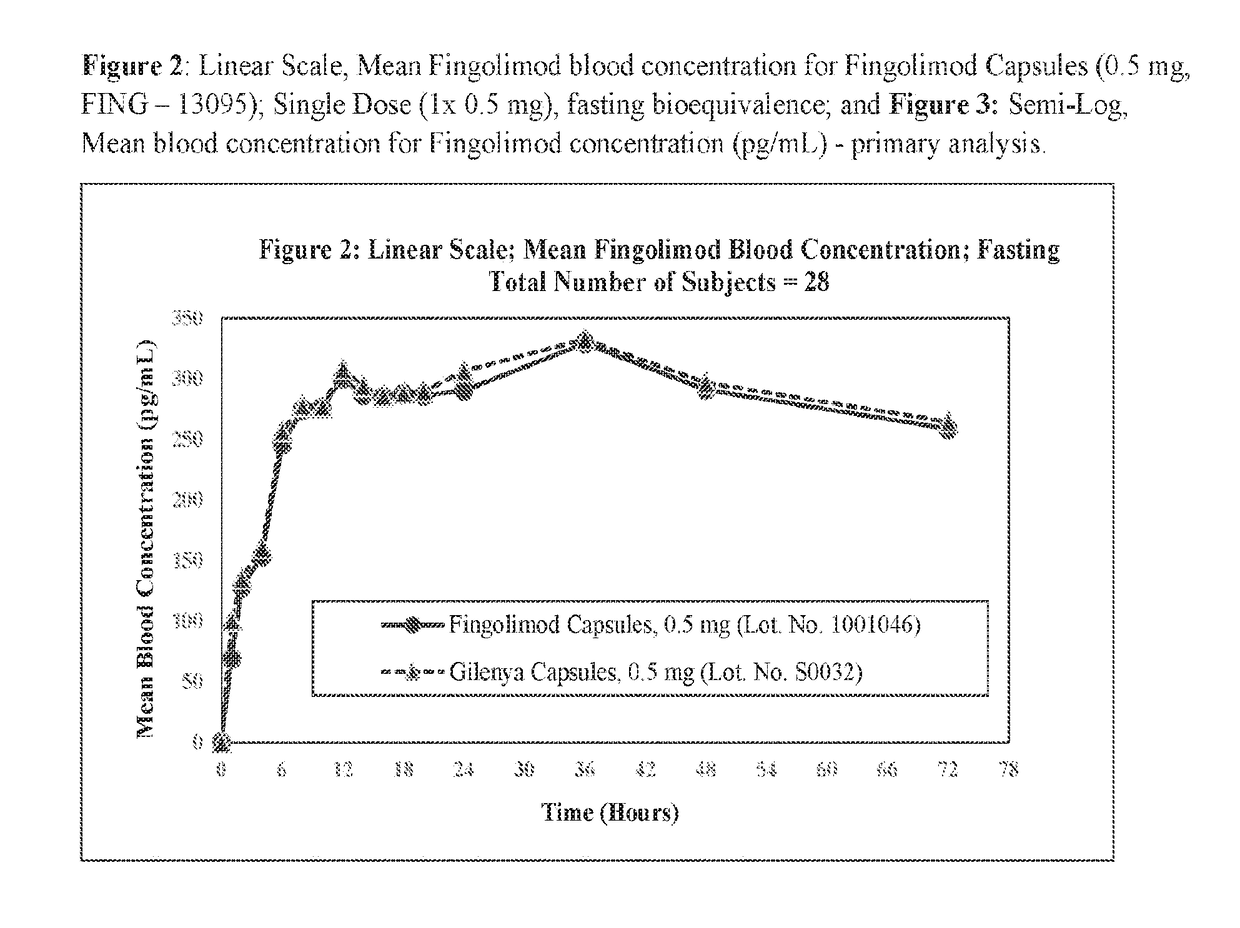
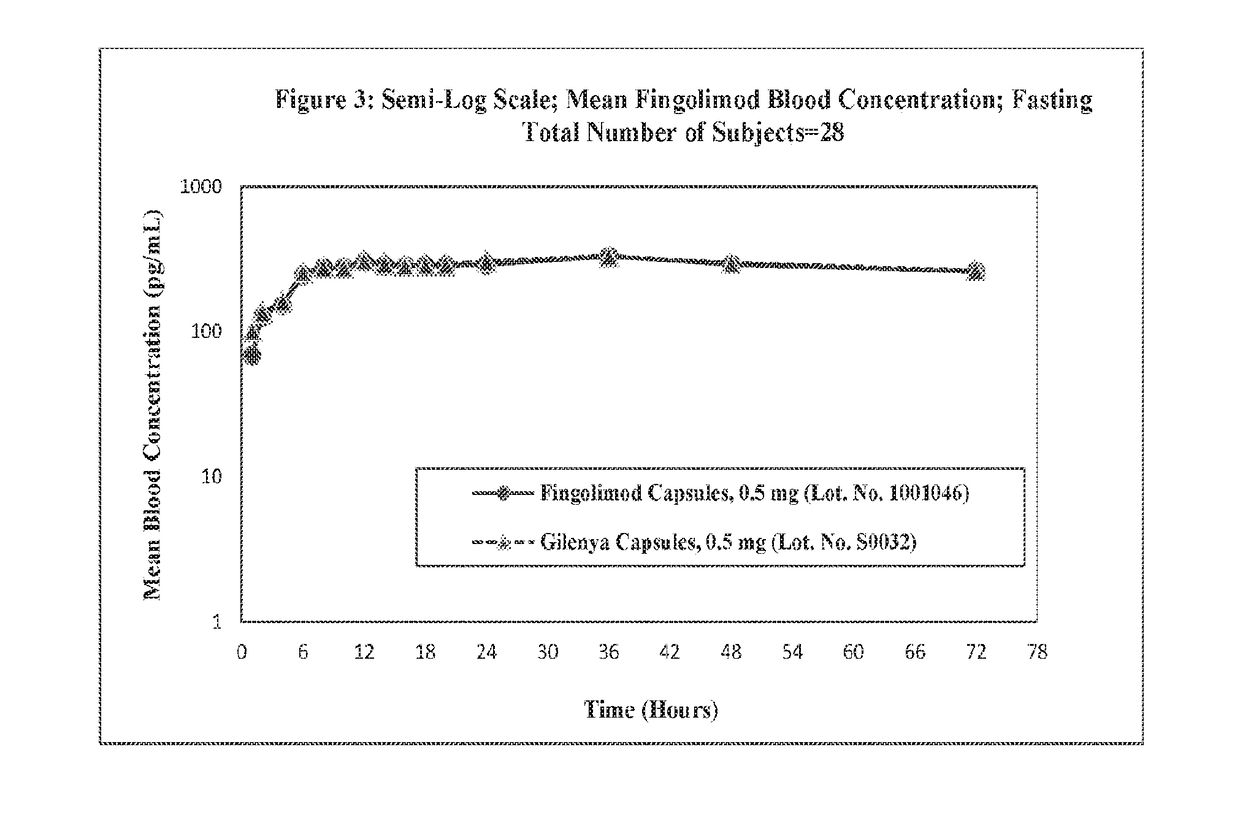
Stable formulations of fingolimod
InactiveUS20180280322A1Improve workabilityImprove uniformityOrganic active ingredientsGranular deliveryGlycineWater insoluble
Sugar alcohol-free formulations of fingolimod. Compositions of fingolimod, salts thereof, or esters thereof that lack sugar alcohols are disclosed. The composition may include a water-soluble filler and a water-insoluble filler, in addition to other common excipients. In some examples, the water-soluble filler is glycine and the water-insoluble filler is dibasic calcium phosphate dihydrate as fillers. In some examples, the water-soluble filler and water-insoluble filler are present in equal concentrations. The compositions disclosed here may be used to make immediate release dosage forms containing fingolimod hydrochloride.
Owner:MYLAN
Development for tanshinol-naringenin composite pellet
InactiveCN106474091APromote dissolutionGuaranteed uniformityOrganic active ingredientsPharmaceutical delivery mechanismEnterohepatic circulationSalvia miltiorrhiza
The invention, which belongs to the medicine field, relates to development for a tanshinol-naringenin composite pellet. Tanshinol being a water-soluble component of the root of red-rooted salvia being Chinese medicine is effective in treating angina pectoris. At present, most of tanshinol preparations being available in the market are immediate-release dosage forms that can not satisfy the demand of long-term therapy for angina pectoris; the bioavailability, being lower than 10%, of the preparation is low and the metabolism is fast, wherein t1 / 2 is equal to 0.5h approximately; and the pure sustained-release pellet can not keep enough blood concentration for long time and thus is not effective in treating angina pectoris. Besides, naringenin is capable of inhibiting activity of P450enzymeCYP3A4; and after taking of the naringenin with tanshinol, the metabolic process of the tanshinol in vivo can be inhibited, so that the bioavailability of tanshinol is enhanced and the plasma clearance is reduced. Therefore, according to development provided by the invention, tanshinol and naringenin are prepared into a composite pellet. In addition, enterohepatic circulation exists during oral naringenin taking and thus a long-acting effect can be realized by an immediate-release dosage form, so that the naringenin is prepared into an immediate-release pellet and the tanshinol is prepared into a sustained-release pellet.
Owner:CHINA PHARM UNIV
Sip modulator immediate release dosage regimen
Owner:NOVARTIS AG
Pharmaceutical compositions of apremilast
The present invention relates to a disintegrant free pharmaceutical composition comprising apremilast or pharmaceutically acceptable salts thereof and one or more pharmaceutically acceptable excipients. Particularly the present invention relates to disintegrant free immediate release dosage form comprising apremilast or pharmaceutically acceptable salts thereof and one or more pharmaceutically acceptable excipients. It further relates to process of preparing such composition and its use in psoriatic arthritis and psoriasis.
Owner:UNICHEM LAB LTD
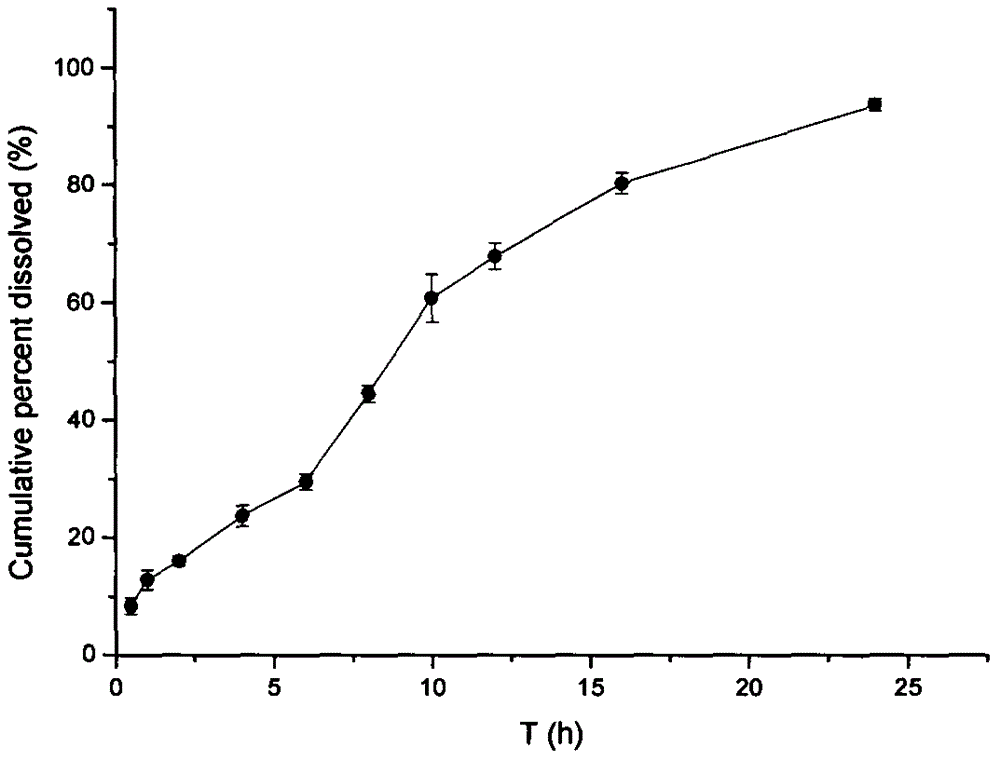
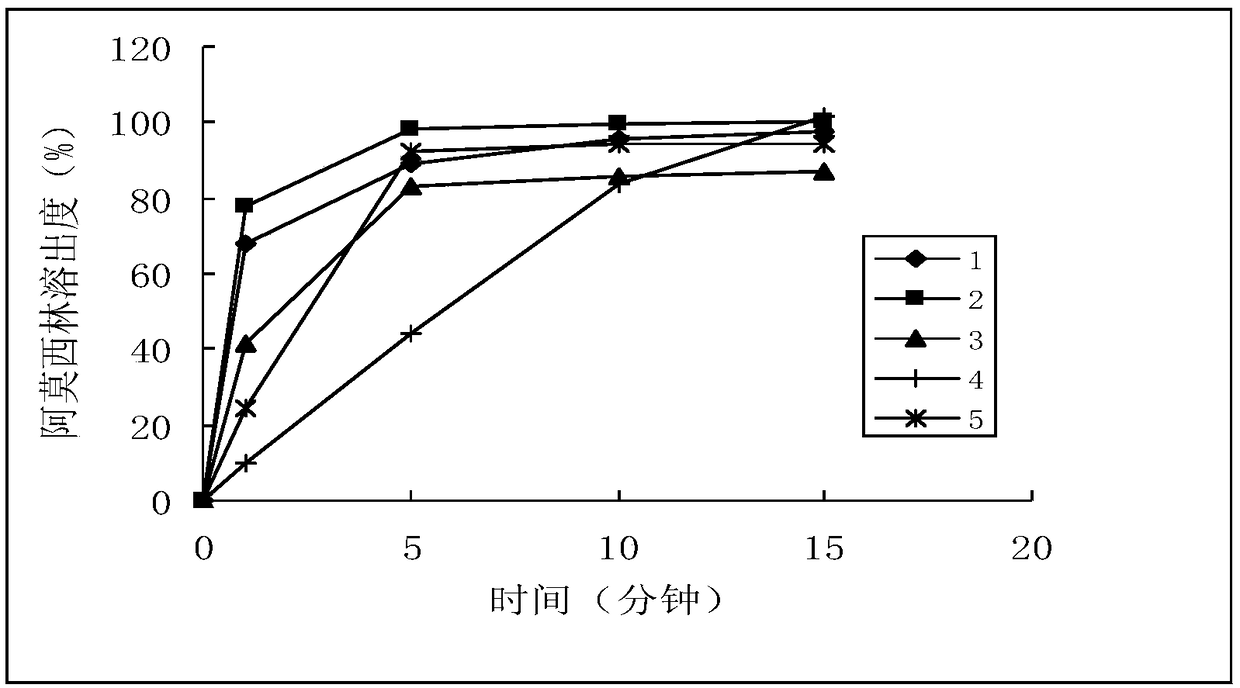
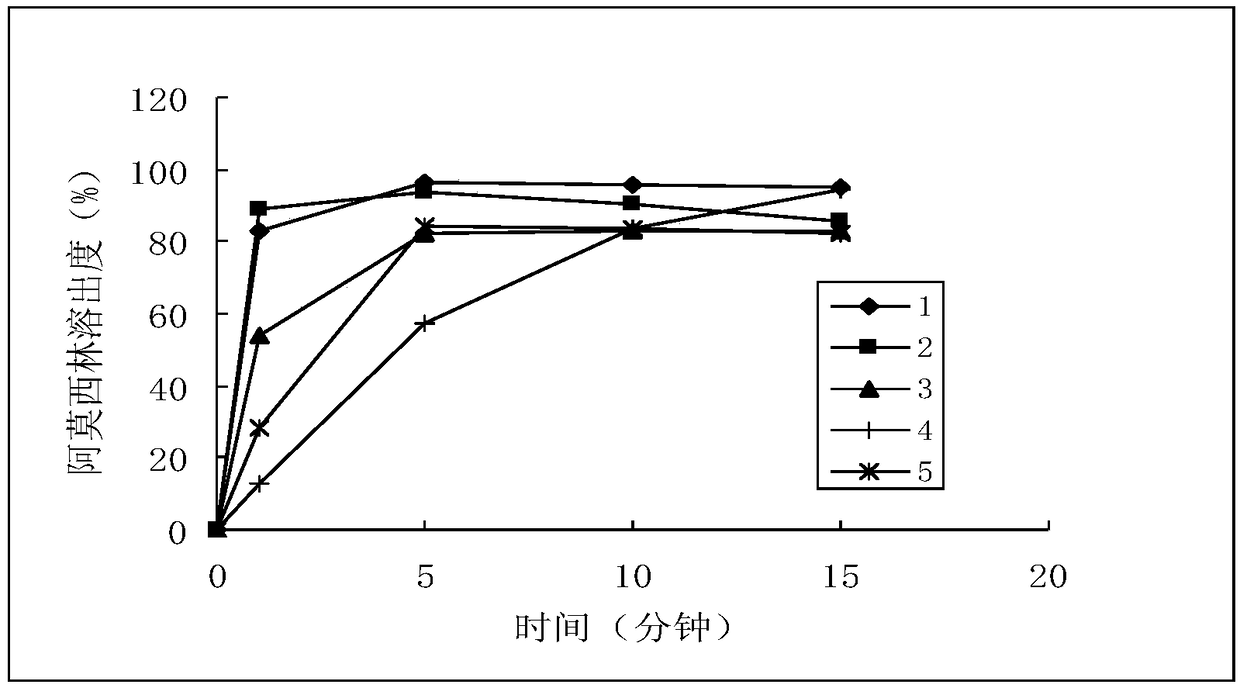
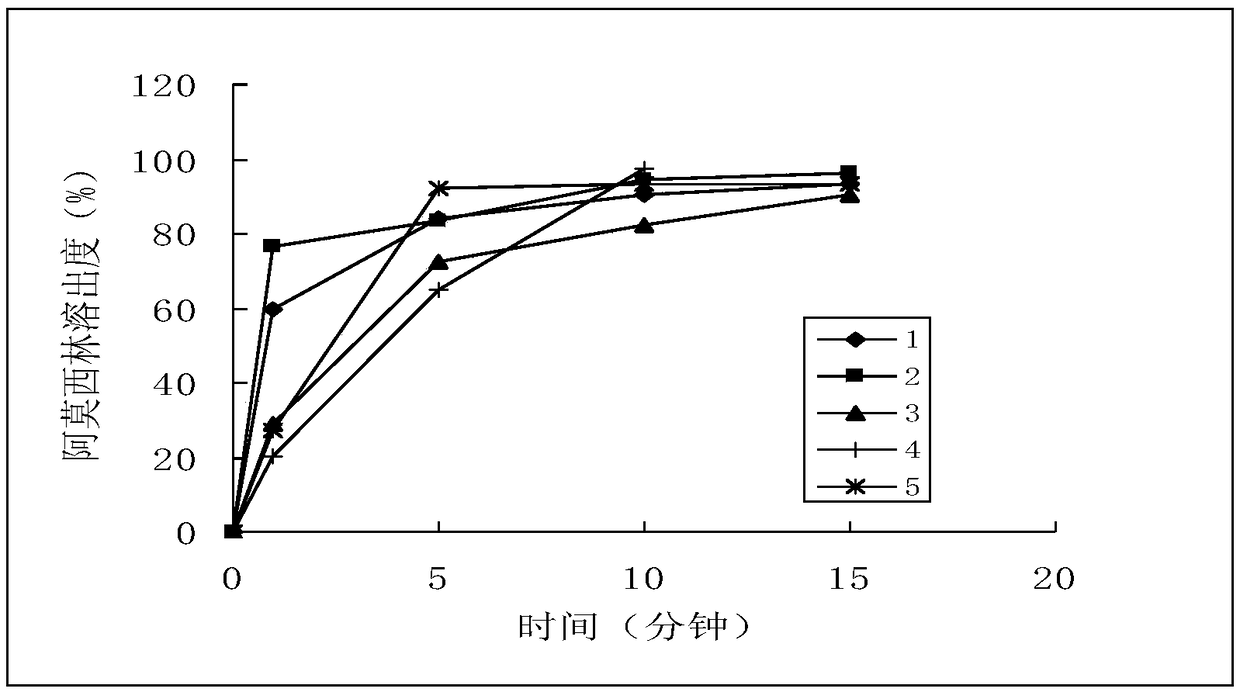
A method for testing the dissolution profile of immediate-release dosage form drug amoxicillin-clavulanate potassium dispersible tablet
ActiveCN106645481BQuality assuranceAchieve consistencyComponent separationReference sampleAmoxicillin-clavulanate potassium
The invention belongs to the field of analytical chemistry, in particular to a method for testing the dissolution curve of amoxicillin-clavulanate potassium dispersible tablets. (1) Through the tests of system applicability, solution stability, linearity, and filter membrane influence factors, etc., determine the test method for high performance liquid phase detection of dissolution; (2) Through the tests of dissolution medium, stirring speed and sampling time point, etc. , to determine the detection method of the dissolution curve; (3) adopt the dissolution curve determination method; adopt the comparative dissolution curve similarity f 2 The factor carries out the comparison of reference sample (sample 1) and 2,3,4,5 sample dissolution curves, and realizes the present invention. The present invention has the advantages of establishing a discriminative dissolution test method, which can well distinguish the impact of the prescription or process variables on the in vitro release of the immediate-release dosage form drug amoxicillin-clavulanate potassium dispersible tablet, thereby ensuring the quality of the drug , to achieve the consistency of quality and efficacy.
Owner:NORTHEAST PHARMA GRP SHENYANG SHIDE PHARMA
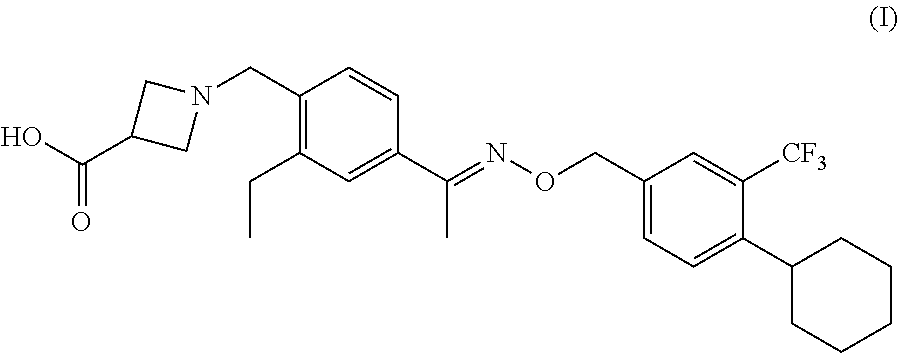
New oral immediated release dosage form
The present invention relates to a solid oral immediate release dosage form of a pharmaceutically active compound, N-[(1,2,3,4-tetrahydro-5-methyl-8-(4-methylpiperazin-1-yl)-2-naphthyl]-4-morpholinobenzamide, in the form of the free base or pharmaceutically acceptable salts thereof. The invention further relates to processes for preparing said dosage form, the use of said dosage form and a method of prevention and / or treatment of CNS disorders and related medical disturbances using said dosage form.
Owner:ASTRAZENECA AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com