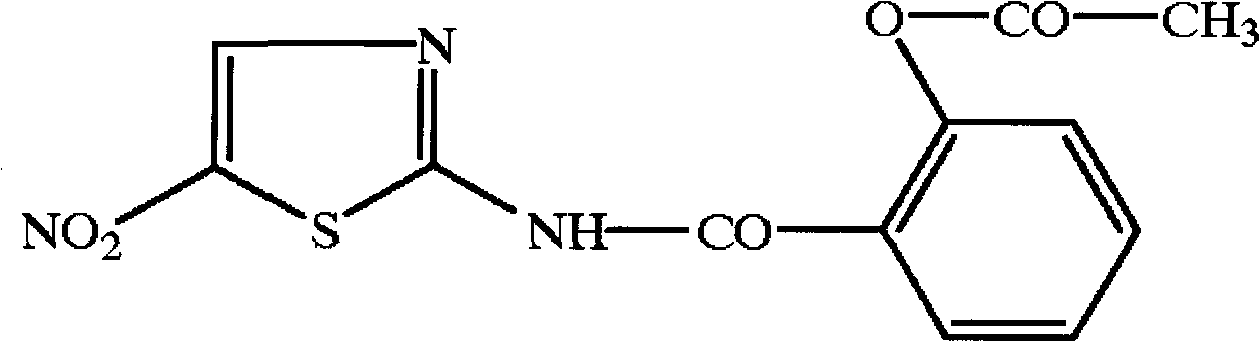
Controlled release pharmaceutical formulations of nitazoxanide
A controlled release agent, nitazoxanide technology, applied to solid dosage forms of nitazoxanide and nitazoxanide analogues, for the treatment of patients with hepatitis C, a field of patients, capable of addressing nitazoxanide The absorption of tezoxanide and tizoxanide is variable, side effects, and the mechanism of action of nitazoxanide is not clear, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Factors affecting the bioavailability of nitazoxanide
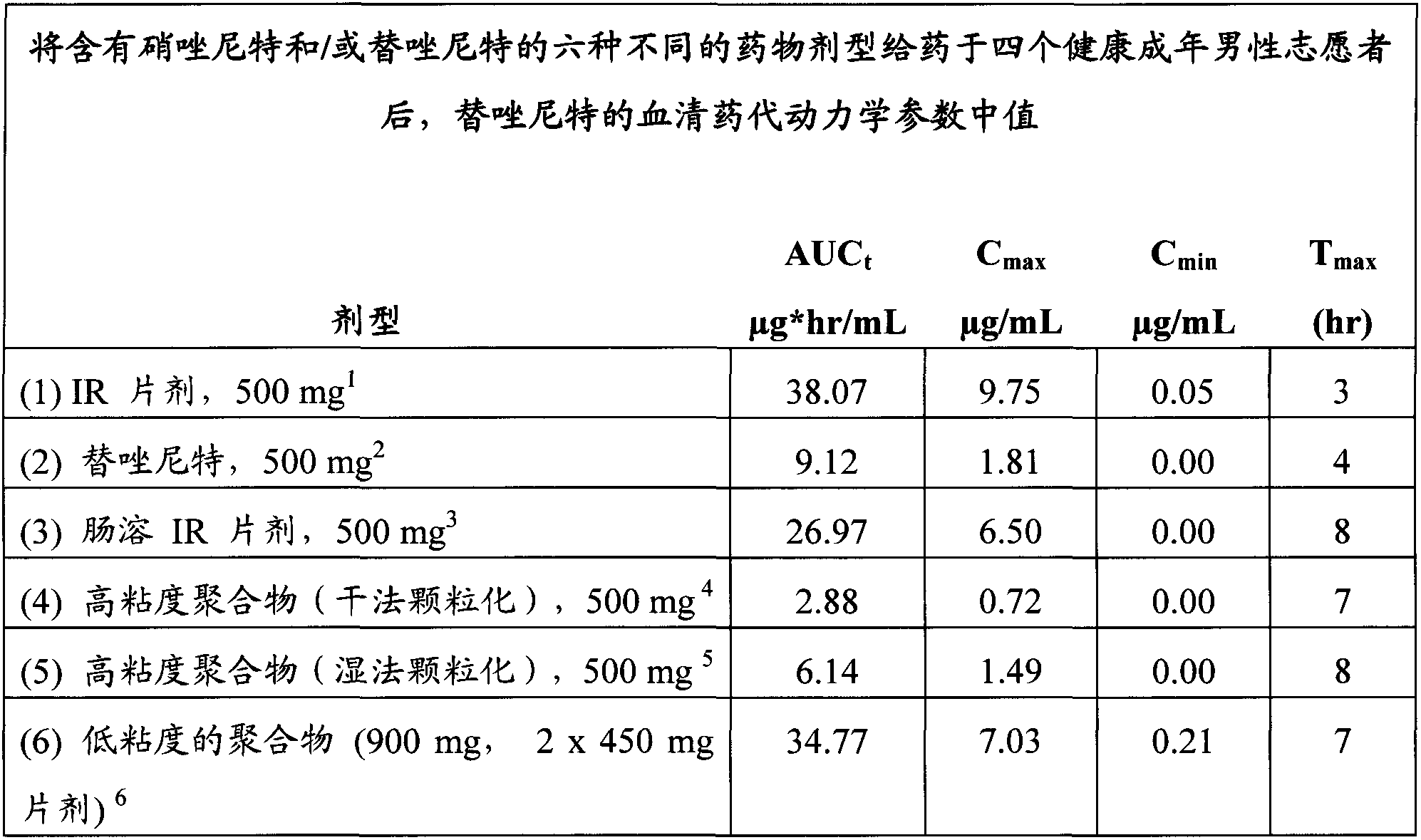
[0065] In order to investigate the impact of the following factors on the bioavailability of nitazoxanide, the present invention has been studied: (1) the absorption of tizoxanide is compared with the absorption of nitazoxanide, (2) changes in the absorption of nitazoxanide in the gastrointestinal tract Site of release, (3) effect of different polymers, and (4) effect of granulation in alcohol versus water.
[0066] To study each factor, six different nitazoxanide and / or tizoxanide formulations were orally administered with food to four healthy adult male volunteers. Each volunteer received each of the six dosage forms in six different treatment periods with at least one week between each treatment period. The dosage form is administered orally with food. Blood samples were collected at the following eleven time points: just before dosing and 1, 2, 3, 4, 5, 6, 7, 8, 10, and 12 hours after dosing. Administer th...
Embodiment 2
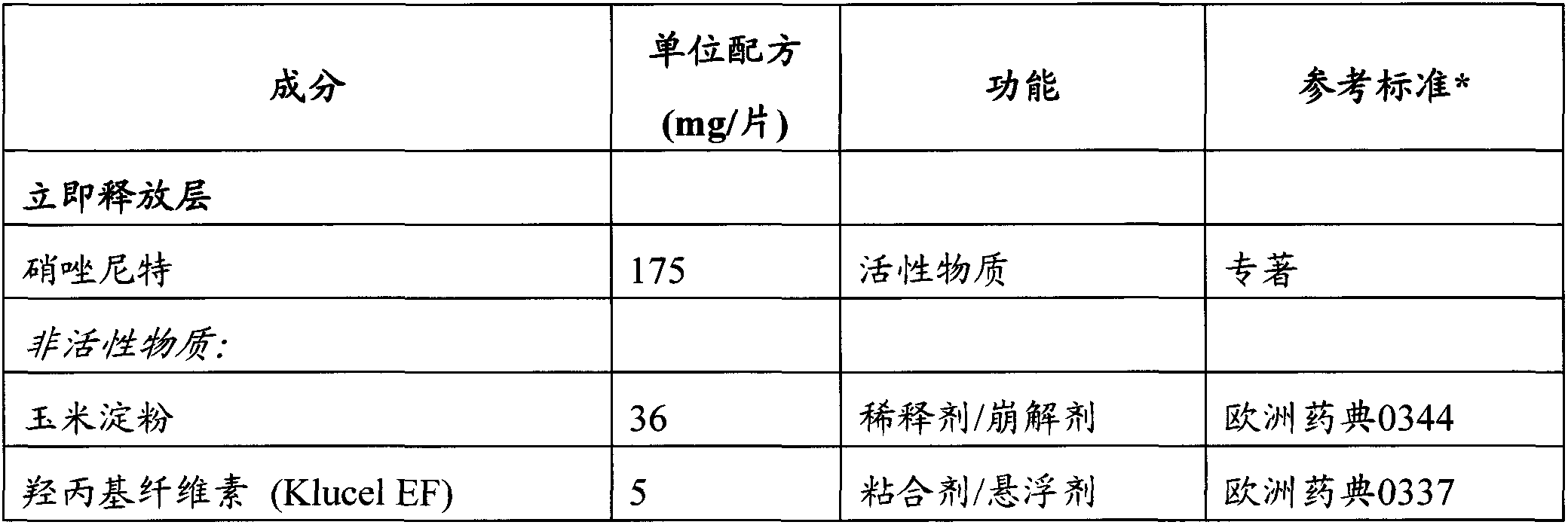
[0088] bilayer tablet dosage form
[0089] Bilayer tablets containing a total of 650 mg of nitazoxanide were manufactured using standard dosage form techniques as previously described. The composition of the bilayer tablet is listed in Table 2.
[0090] Table 2: Composition of 675 mg Nitazoxanide Controlled Release Tablets.
[0091]
[0092]
[0093] * Refer to the current version.
Embodiment 3
[0095] Batch formulation of bilayer tablets
[0096] As indicated in Table 3, a batch of 100,000 bilayer tablets of nitazoxanide of Example 2 (650 mg) was prepared.
[0097] Table 3. Batch production formulation of 675mg nitazoxanide controlled release tablet
[0098]
[0099]
[0100] Tablets were manufactured following the manufacturing protocol outlined below.
[0101] a. equipment
[0102] Frewitt Sieve
[0103] Collette Planetary Blender
[0104] oven
[0105] Tablet Press - Manesty BB Press
[0106] tablet duster
[0107] Coating Equipment-Accelacota
[0108] Clean all production equipment before use.
[0109] b. Preparation of Immediate Release Granules (Granule A)
[0110] 1. Weigh the raw material and put it into a sealed plastic bag.
[0111] 2. Check the integrity of the machine before and after use.
[0112] 3. If necessary, nitazoxanide and cornstarch were sifted through a sieve with a mesh size of 1.25 mm using a Frewitt machine.
[0113] 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com