Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an anti-cancer agent
a methotrexate and antirheumatic drug technology, applied in the field of sustained release formulations of methotrexate, can solve the problems of low bioavailability than subcutaneous administration, and difficulty in controlling csub>max /sub> with conventional dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
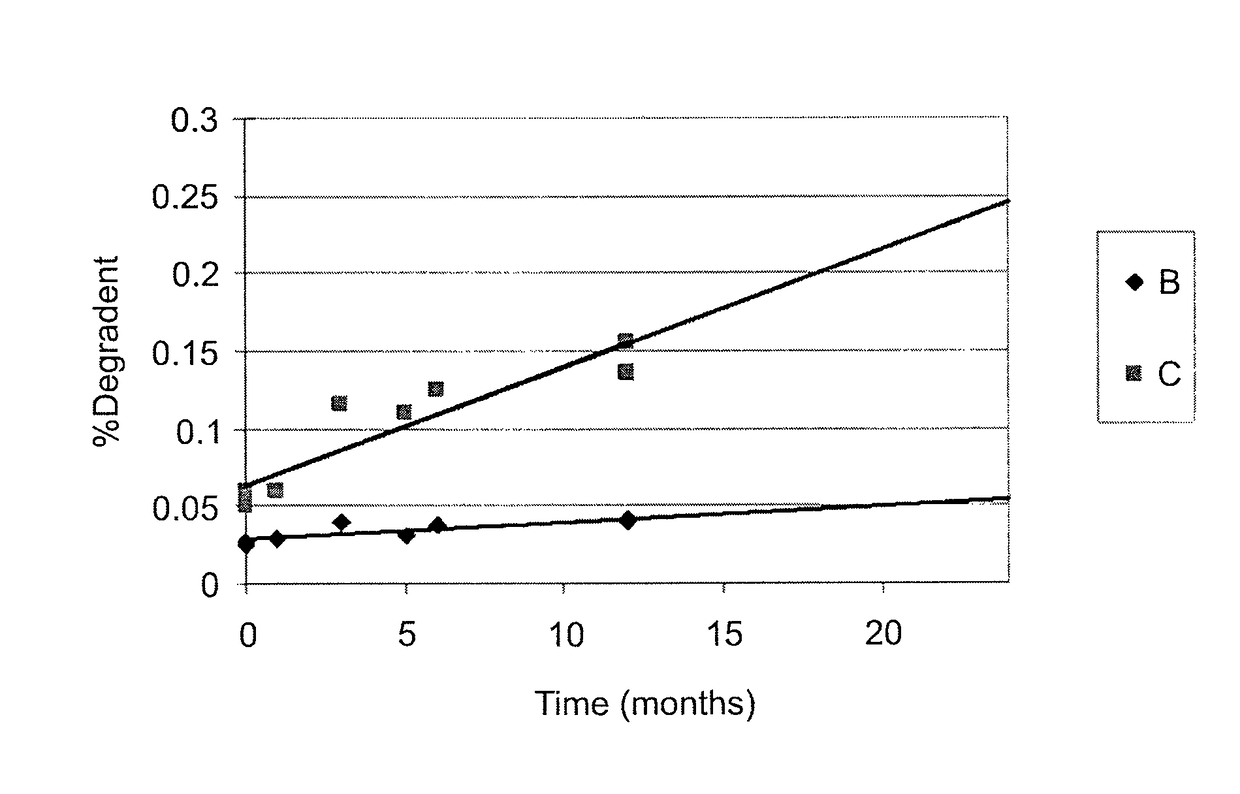
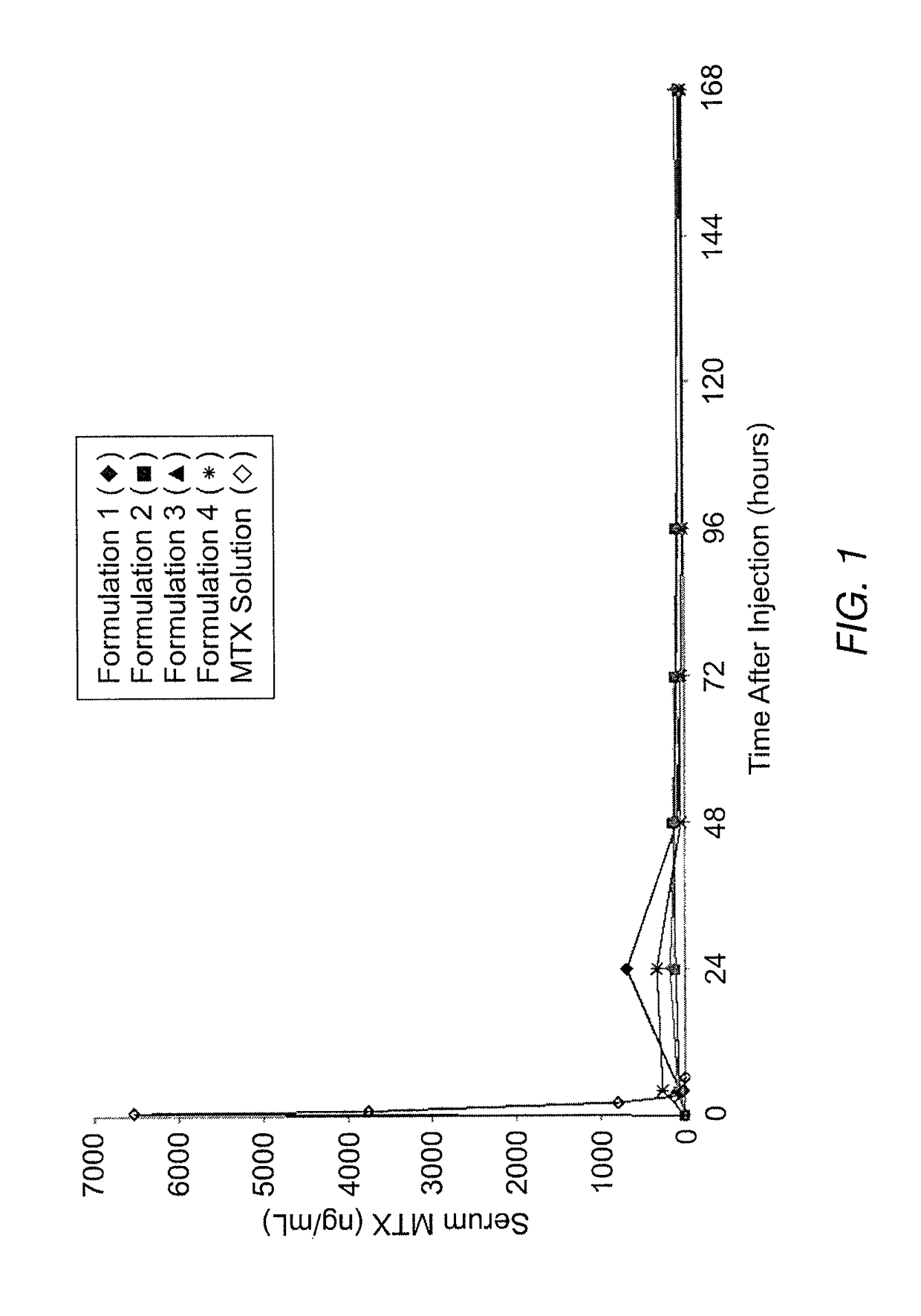
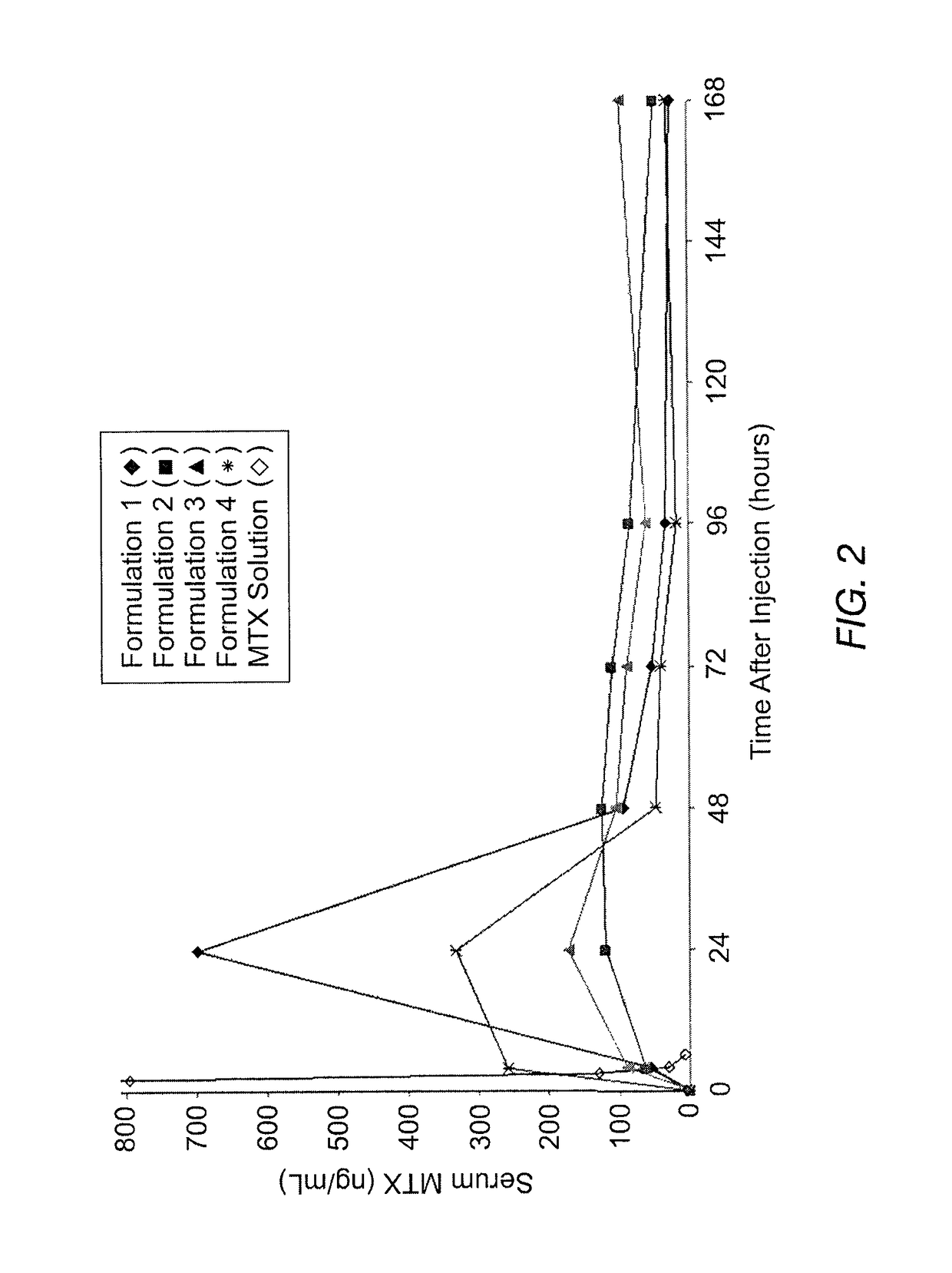
[0066]The instant MVL MTX formulations were prepared in a manner similar to that reported by Kim et al. (Biochim Biophys Acta, 728 (1983) 339-348). An aqueous solution containing MTX, sodium hydroxide and sucrose was emulsified with chloroform solution containing DOPC or DEPC, DPPG, tricaprylin and / or triolein, and cholesterol, resulting in a water-in-oil (W / O) emulsion. The W / O emulsion was then emulsified in a second aqueous solution containing lysine and sucrose to produce a W / O / W emulsion. The W / O / W emulsion was then was stirred at 37° C. under a nitrogen stream to remove the chloroform by evaporation. The resulting particles were centrifuged, and the supernatant replaced with normal saline. After washing, the particles were diluted into normal saline to yield a product with approximately a 50% packed particle volume (PPV). PPV is the fraction of the total formulation volume taken up by the MVL particles.
[0067]The compositions of the solutions used to prepare four formulations b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com