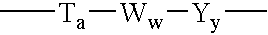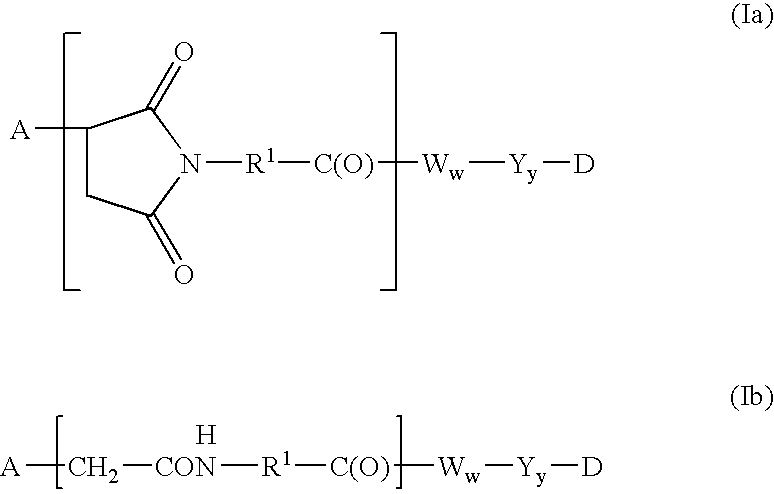Patents
Literature
1235 results about "Immunologic diseases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An immunological disease results from the disorder of the immune system. It may fall into one of these categories: allergic diseases, autoimmune diseases, immune complex diseases, and immunodeficiency disorders.
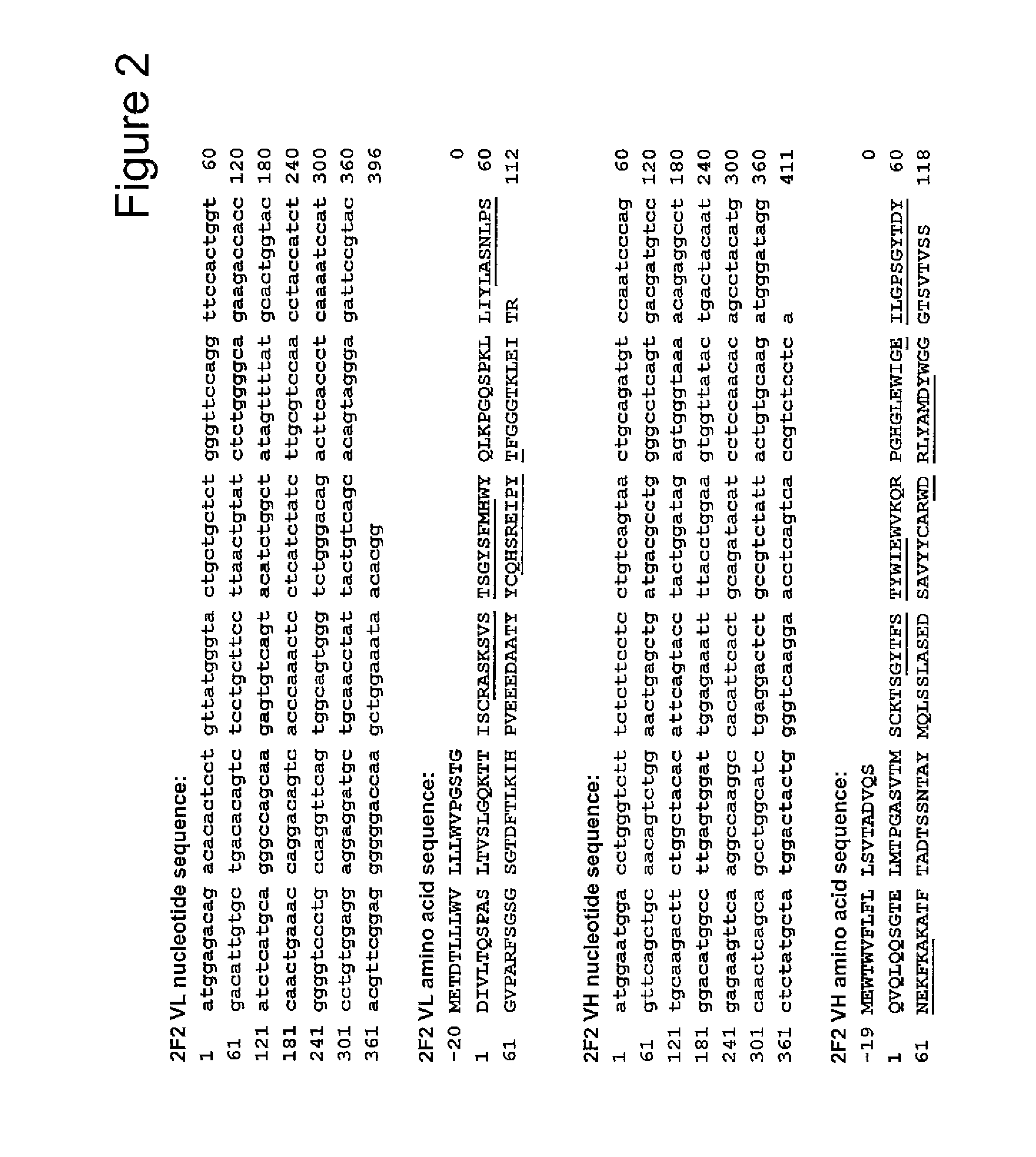
Heterocyclic compounds
InactiveUS6329381B1Excellent interferon biosynthesis inducing activityInhibition thicknessAntibacterial agentsBiocideBULK ACTIVE INGREDIENTInterferon inducer
The present invention relates to a heterocyclic compound of the following general formula (I):wherein X is sulfur atom, oxygen atom or -NR3- (R3 may form a heterocyclic ring or a substituted heterocyclic ring with R1 via the nitrogen atom),R1 is alkyl group, substituted alkyl group, aryl group, substituted aryl group, heterocyclic group or substituted heterocyclic group, andR2 is hydrogen atom, halogen atom etc.;or its pharmaceutically acceptable salt and interferon inducers, antiviral agents, anticancer agents and therapeutic agents for immunologic diseases comprising the compound (I) or its pharmaceutically acceptable salt as active ingredients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
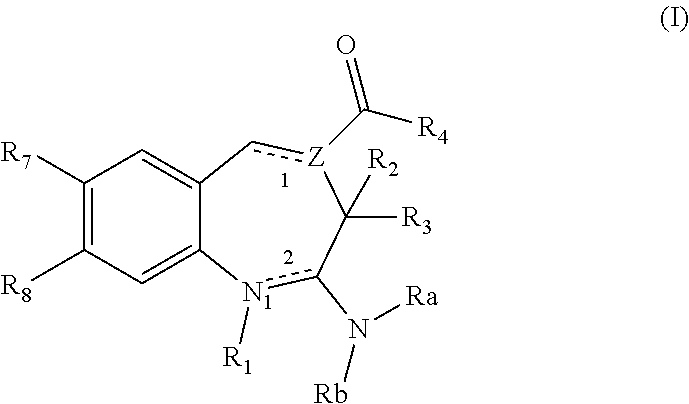
Substituted Benzoazepines As Toll-Like Receptor Modulators
Provided are compositions and methods useful for modulation of signaling through the Toll-like receptors TLR7 and / or TLR8. The compositions and methods have use in treating or preventing disease, including cancer, autoimmune disease, fibrotic disease, cardiovascular disease, infectious disease, inflammatory disorder, graft rejection, or graft-versus-host disease.
Owner:ARRAY BIOPHARMA +1
Injectable compositions of nanoparticulate immunosuppressive compounds
InactiveUS20060210638A1Improve complianceImprove efficacyPowder deliveryBiocideDepressantCompound (substance)
The invention is directed to an injectable nanoparticulate immunosuppressant composition for the formation of a subcutaneous or intramuscular depot. The invention is also directed to an injectable composition of nanoparticulate tacrolimus and / or sirolimus which eliminates the need to use polyoxyl 60 hydrogenated castor oil (HCO-60) and / or polysorbate 80 as a solubilizer. This invention further discloses a method of making an injectable nanoparticulate tacrolimus and / or sirolimus composition and is also directed to methods of treatment using the injectable nanoparticulate formulations comprising tacrolimus, sirolimus, or combination thereof for a subcutaneous or intramuscular depot for the prophylaxis of organ rejection and for the treatment of psoriasis or other immune diseases
Owner:ELAN PHRMA INT LTD
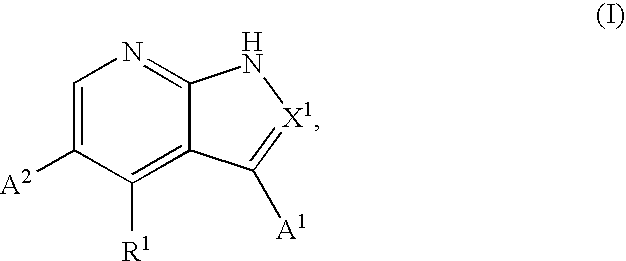
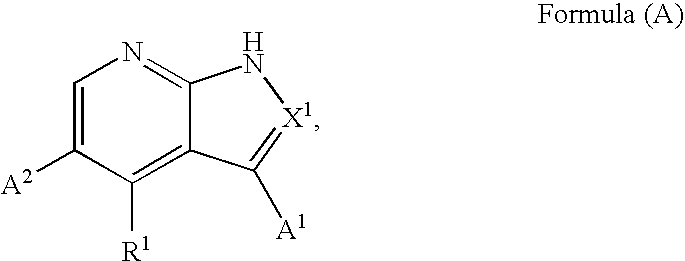
Substituted pyrrolopyridines and pyrazolopyridines as kinase modulators
Provided herein are substituted pyrrolopyridine heterocycles and substituted pyrazolopyridine heterocycles, pharmaceutical compositions comprising said heterocycles and methods of using said heterocycles in the treatment of disease. The heterocycles disclosed herein function as kinase modulators and have utility in the treatment of diseases such as cancer, allergy, asthma, inflammation, obstructive airway disease, autoimmune diseases, metabolic disease, infection, CNS disease, brain tumor, obesity, asthma, hematological disorder, degenerative neural disease, cardiovascular disease, or disease associated with angiogenesis, neovascularization, or vasculogenesis.
Owner:SGX PHARMA INC
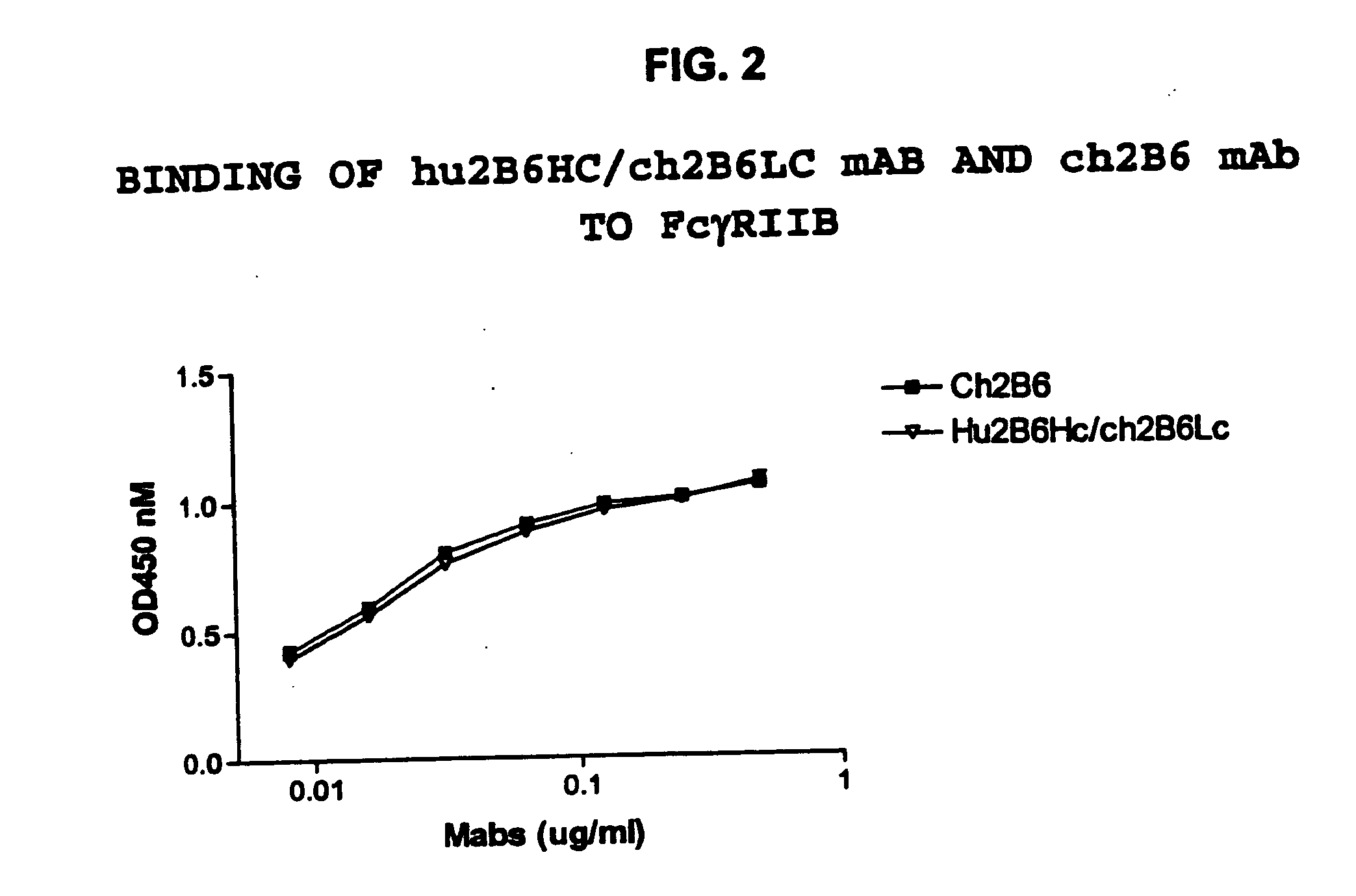
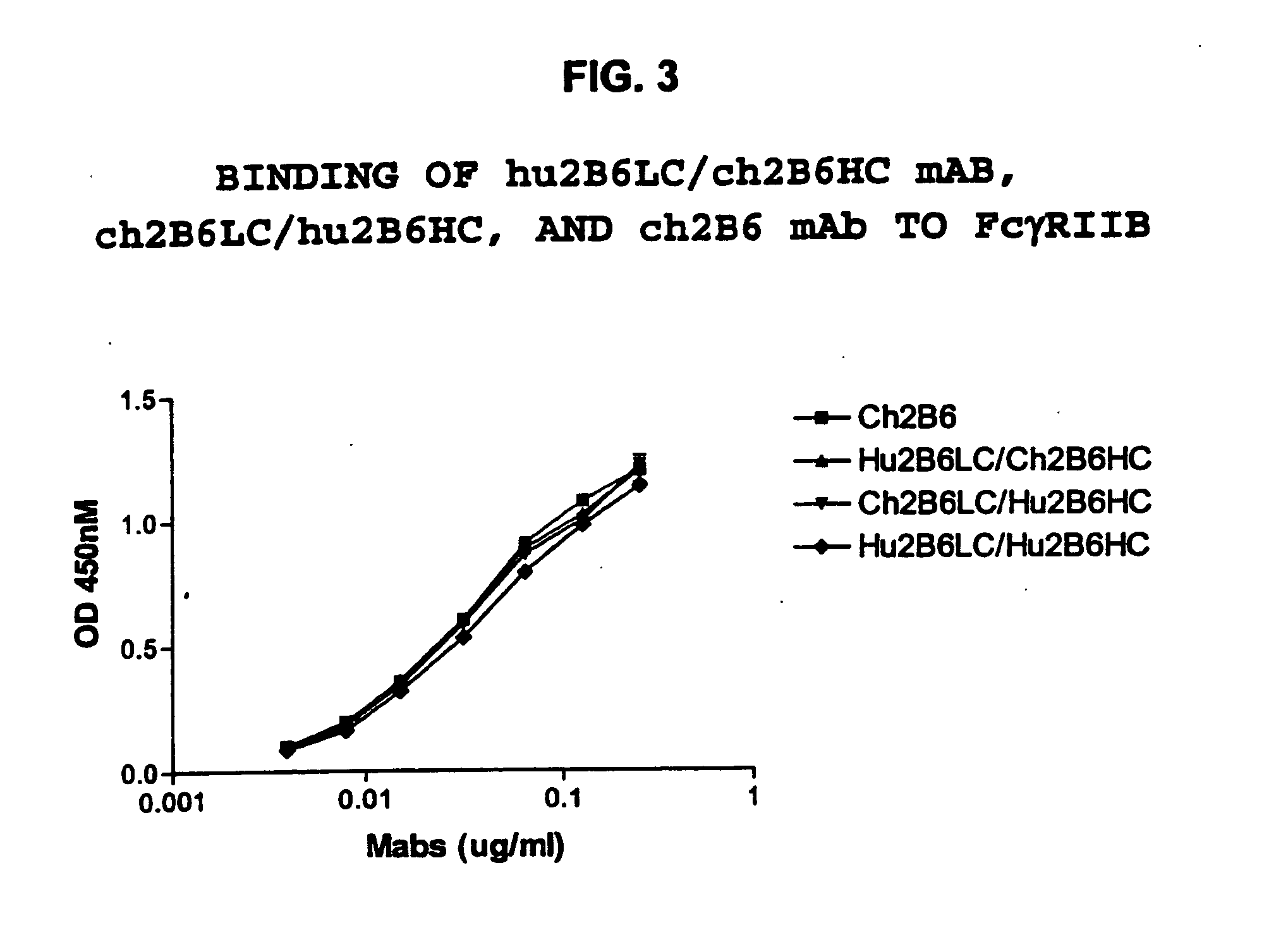
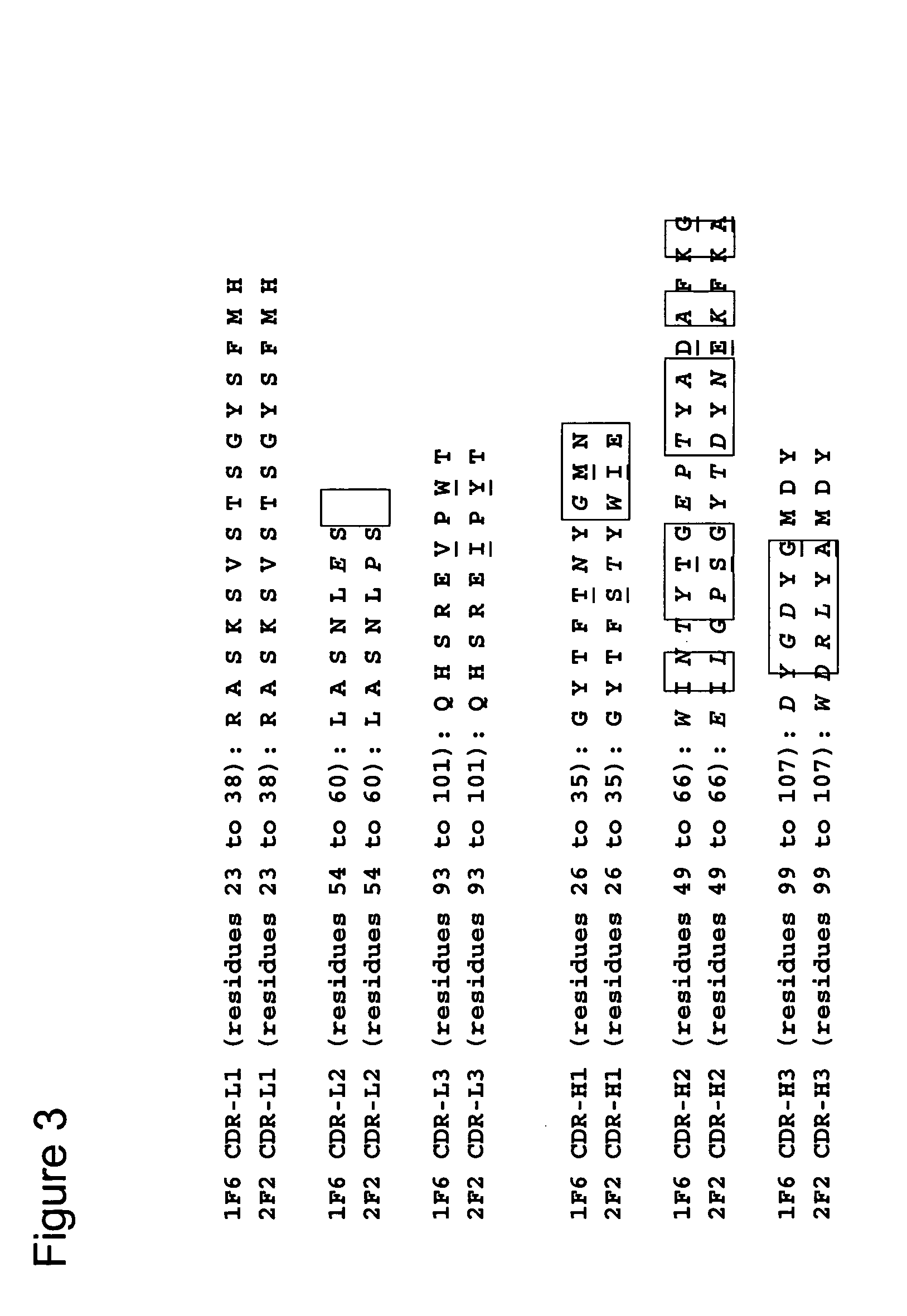
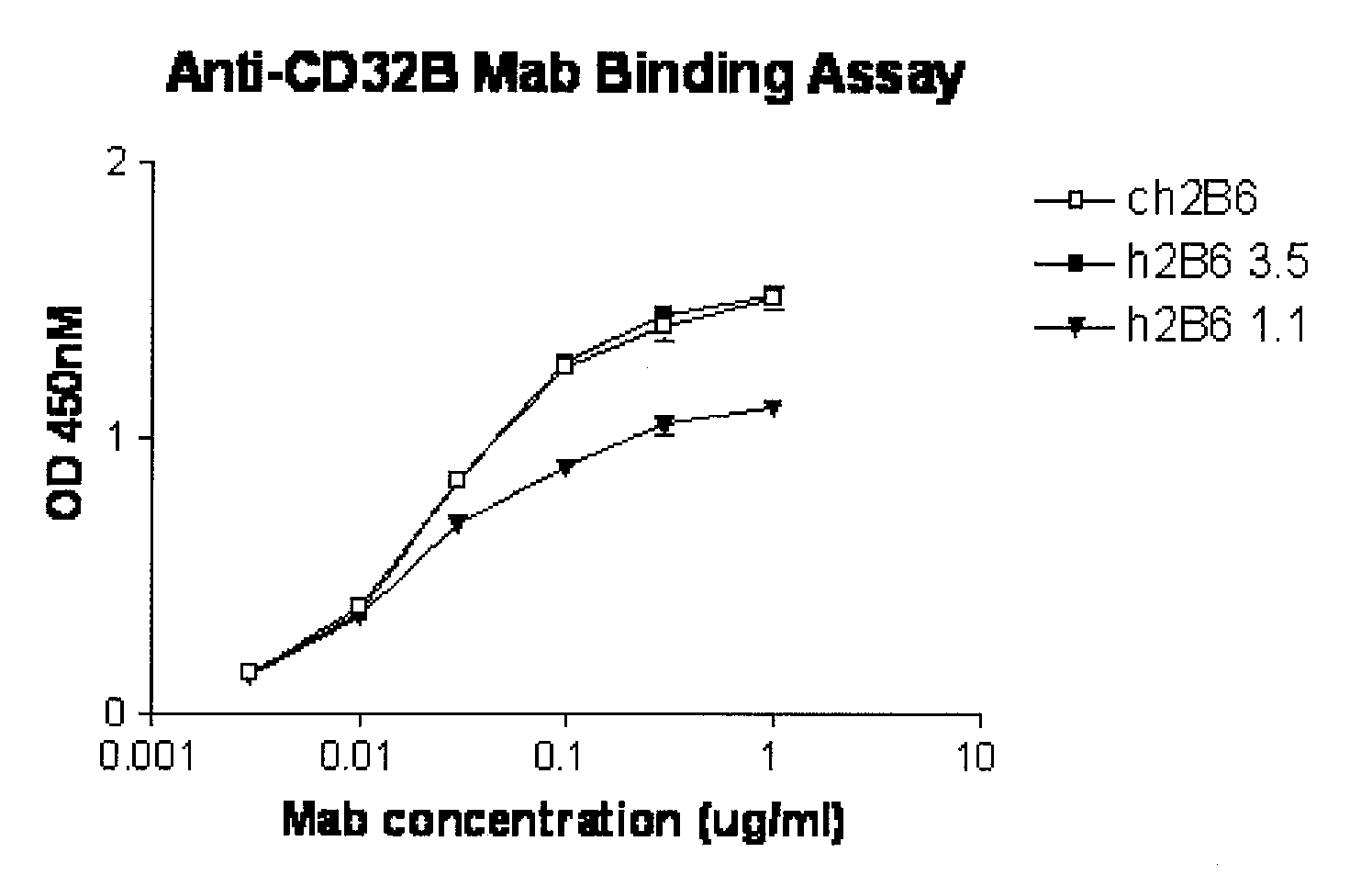
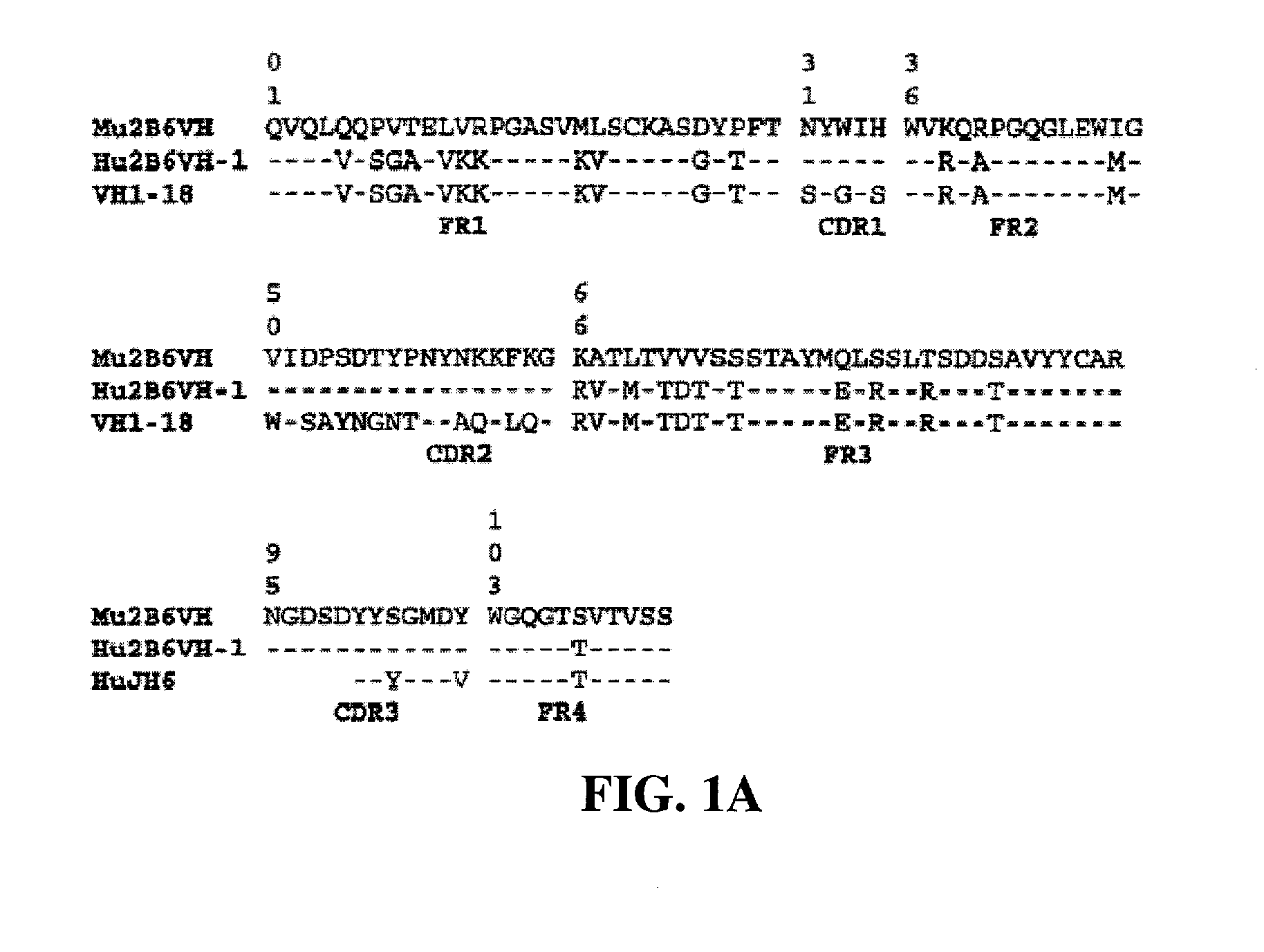
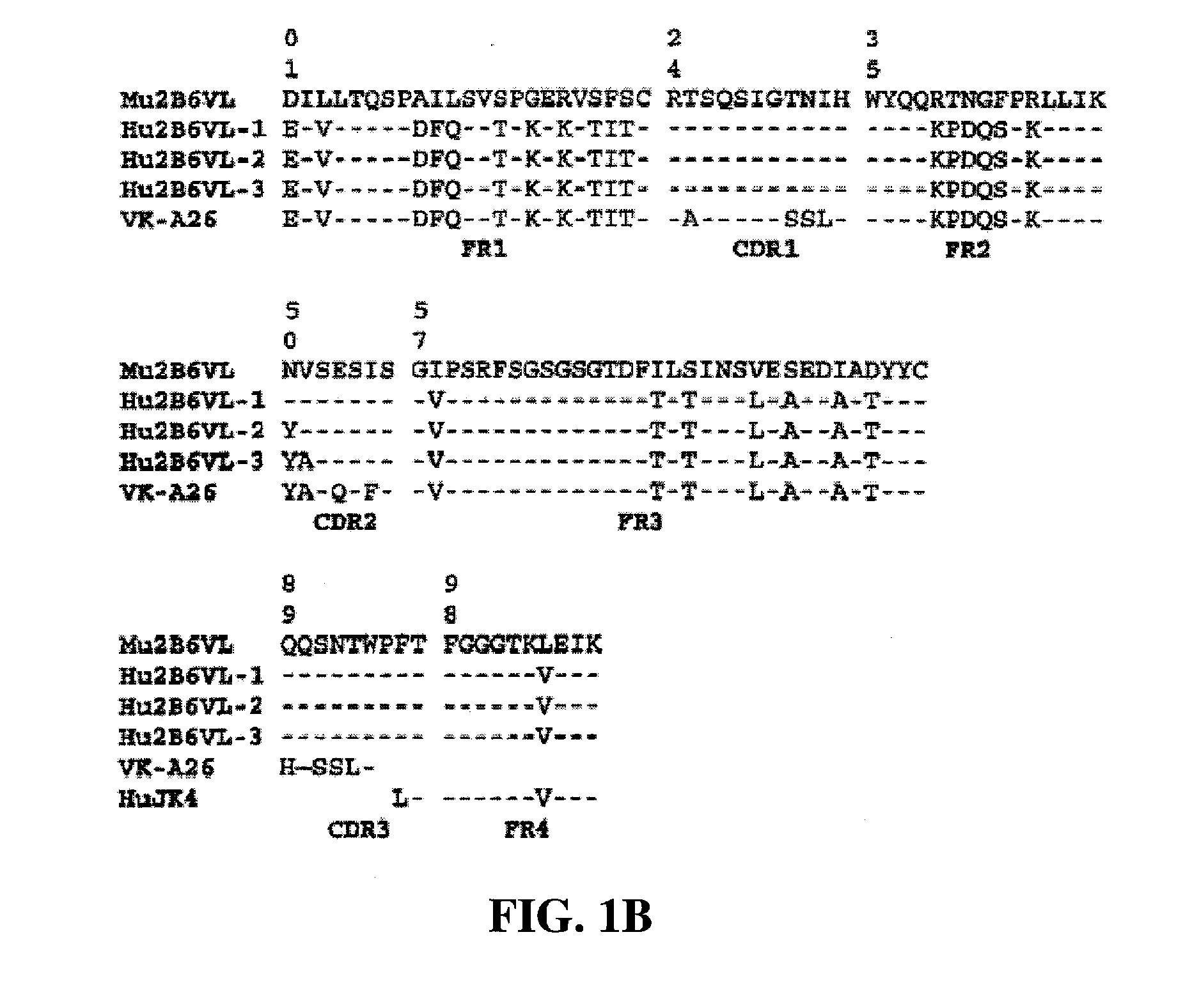
Humanized FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20060013810A1Good curative effectConvenient treatmentSenses disorderNervous disorderFc(alpha) receptorDisease
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
Anti-cd70 antibody-drug conjugates and their use for the treatment of cancer and immune disorders
ActiveUS20060233794A1Prevent relapseAntibacterial agentsNervous disorderDrug conjugationAntiendomysial antibodies
Disclosed are anti-CD70 antibodies and derivatives thereof conjugated to cytotoxic, immunosuppressive, or other therapeutic agents, as well as pharmaceutical compositions and kits comprising the antibody- and antibody derivative-drug conjugates. Also disclosed are methods, for the treatment of CD70-expressing cancers and immunological disorders, comprising administering to a subject the disclosed pharmaceutical compositions.
Owner:SEAGEN INC
Treatment of immunological disorders using anti-dc30 antibodies
InactiveUS20050123536A1Enhancing cytotoxicEnhancing cytostatic effectOrganic active ingredientsSenses disorderDiseaseAntibody conjugate
The present invention relates to methods for the treatment of immunological disorders other than cancer, comprising administering proteins characterized by their ability to bind to CD30 and exert a cytostatic or cytotoxic effect on an activated lymphocyte. Such proteins include monoclonal antibodies AC10 and IleFi1. AC10 and HeFi-1 derivatives, and antibodies that compete with AC10 and HeFi-1 for binding to CD30. Other such proteins include multivalent anti-CD30 antibodies and anti-CD30 antibodies conjugated to cytotoxic agents. Treatment modalities with antibodies of the invention are also provided.
Owner:SEATTLE GENETICS INC
Anti-CD20 antibody-drug conjugates for the treatment of cancer and immune disorders
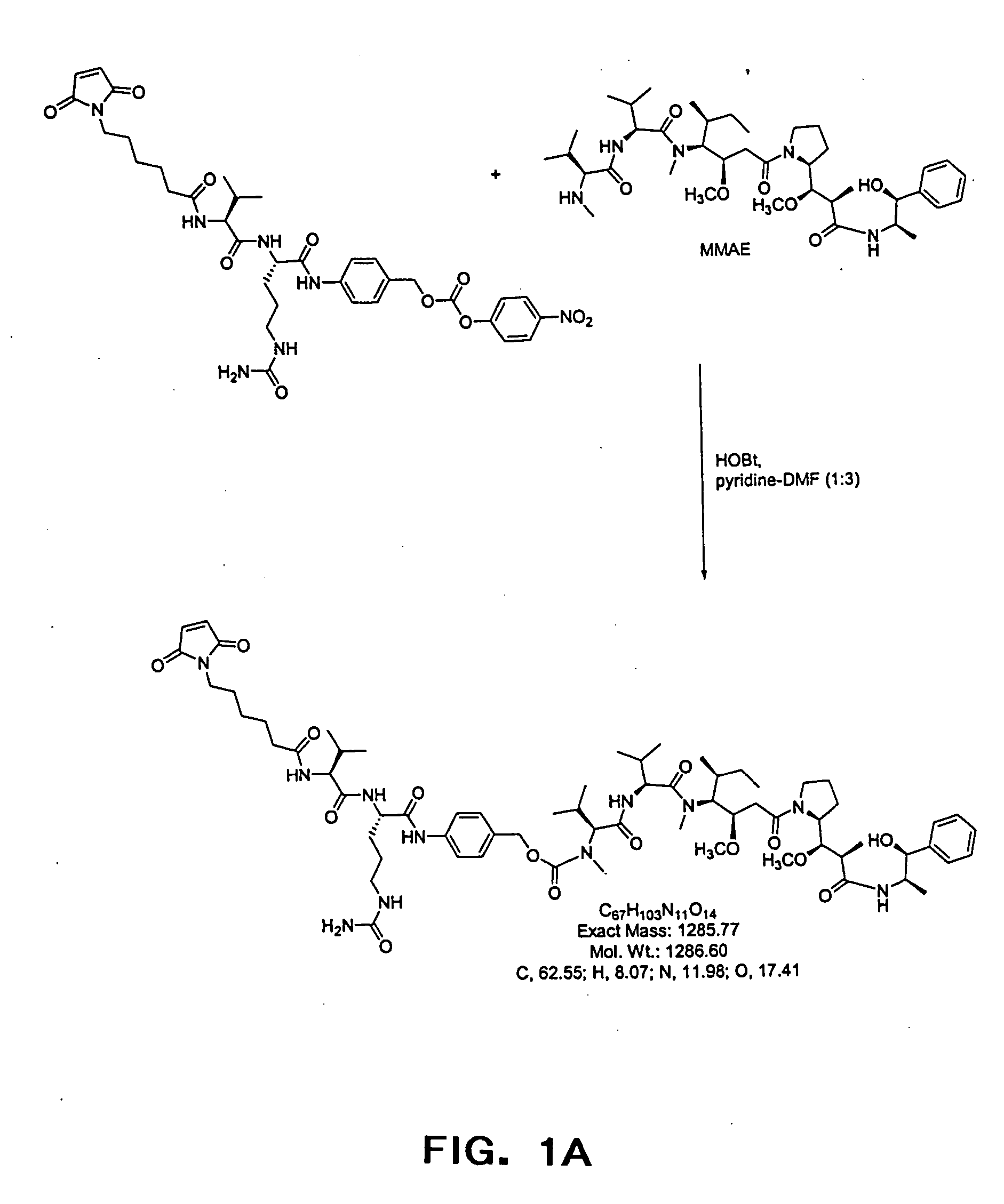
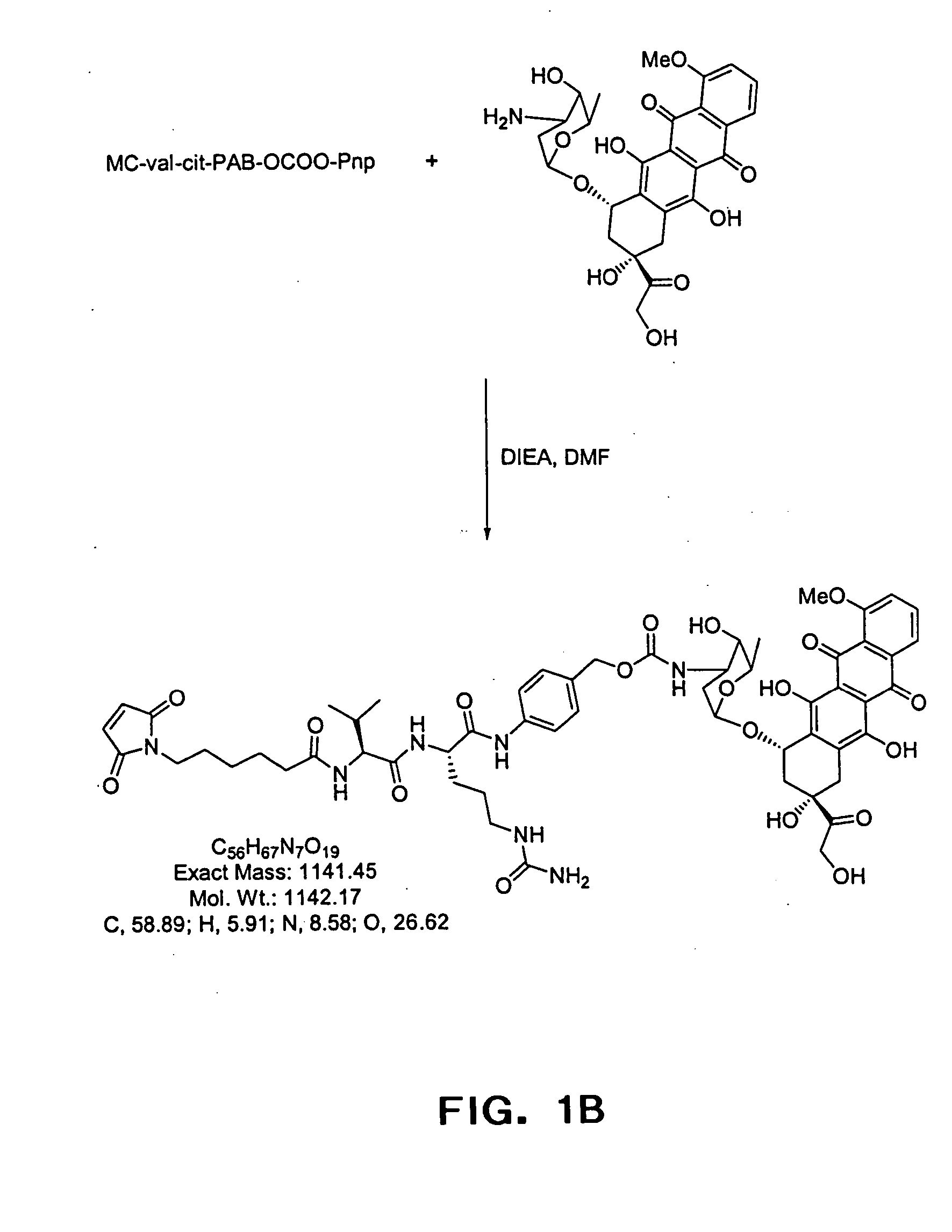
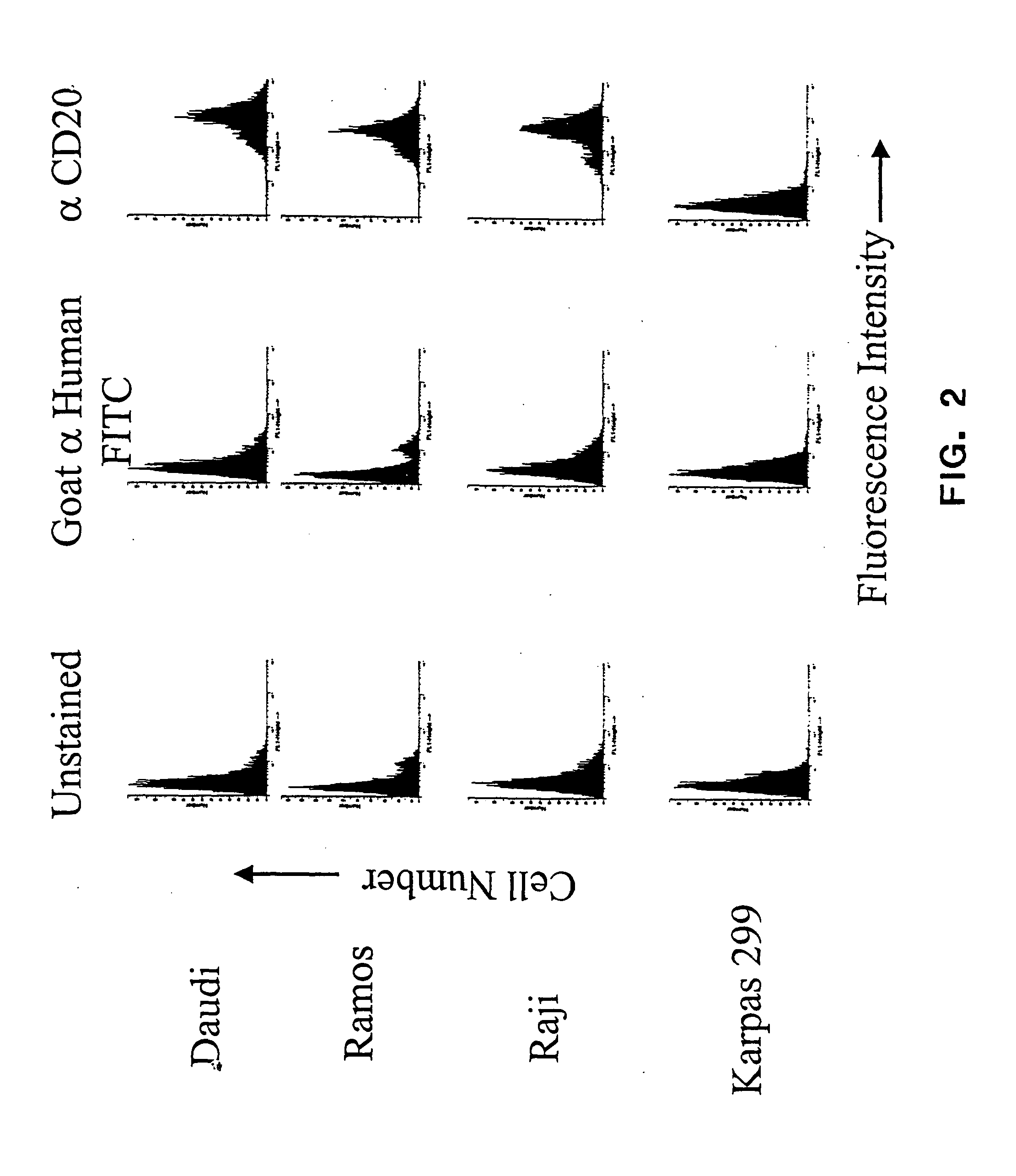
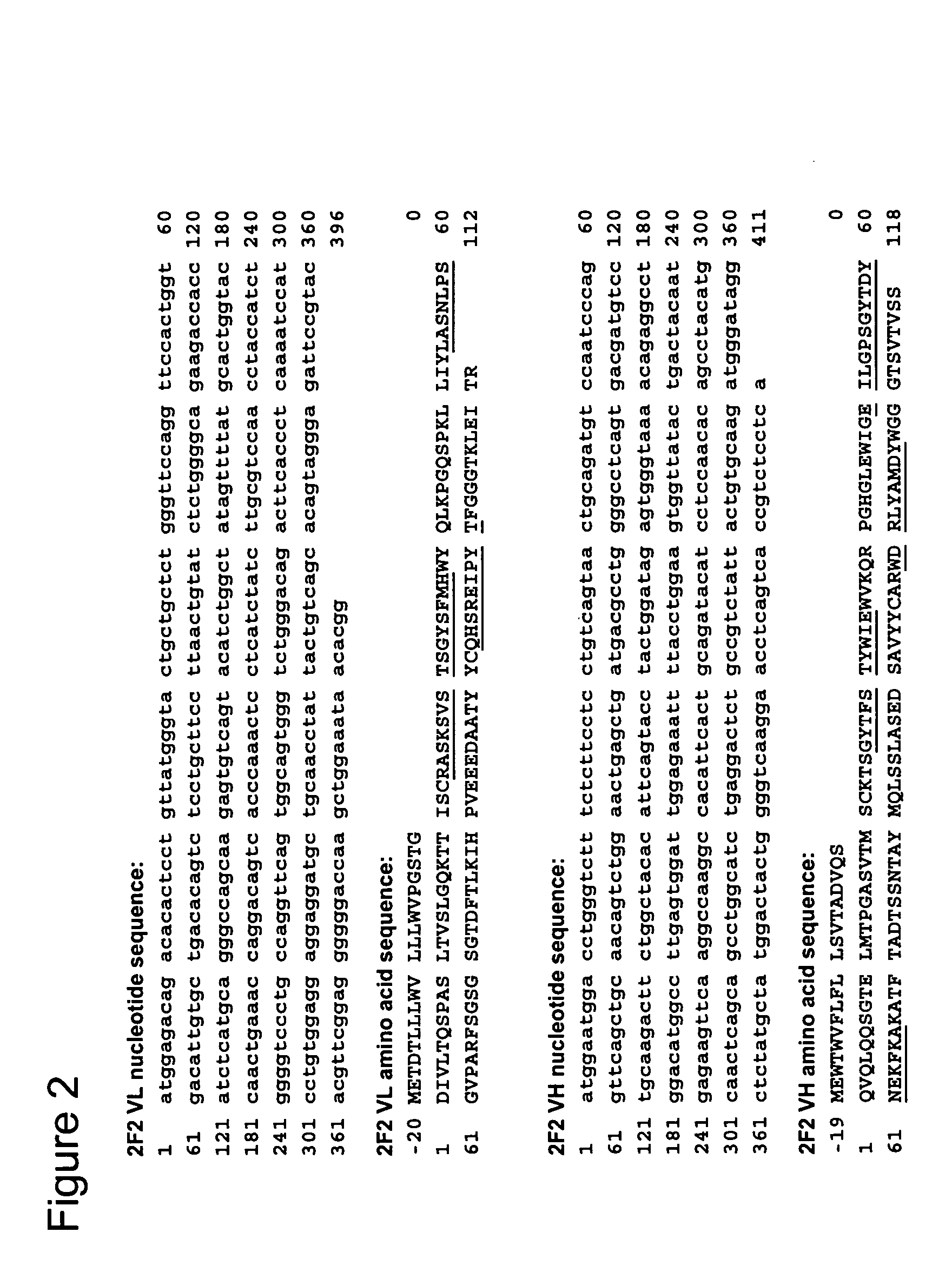
The present invention relates to methods and compositions for the treatment of CD20-expressing cancers and immune disorders involving CD20-expressing cells. The present methods comprise administering to a subject an anti CD20 antibody-drug conjugate that has a high potency and / or is capable of internalizing into CD20-expressing cells. The present invention further provides pharmaceutical compositions and kits comprising such conjugates. The present invention yet further provides methods of and compositions relating to combination therapy of cancer and immune disorders involving CD20-expressing cells using the anti-CD20 antibody-drug conjugates of the invention.
Owner:SEATTLE GENETICS INC
Anti-CD70 antibody and its use for the treatment and prevention of cancer and immune disorders
Disclosed are CD70 binding agents, such as anti-CD70 antibodies and derivatives, that induce a cytotoxic, cytostatic or immunosuppressive without conjugation to a therapeutic agents, as well as pharmaceutical compositions and kits comprising the antibody or derivative. Also disclosed are methods for the treatment and prevention of CD70-expressing cancers and immunological disorders comprising administering to a subject the CD70-binding agent.
Owner:SEAGEN INC
Humanized Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
InactiveUS20080044417A1Good curative effectEnhanced effector functionDisease diagnosisTissue cultureFc(alpha) receptorFc receptor
The present invention relates to humanized FcγRIIB antibodies, fragments, and variants thereof that bind human FcγRIIB with a greater affinity than said antibody binds FcγRIIA. The invention encompasses the use of the humanized antibodies of the invention for the treatment of any disease related to loss of balance of Fc receptor mediated signaling, such as cancer, autoimmune and inflammatory disease. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the humanized antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing the efficacy of a vaccine composition by administering the humanized antibodies of the invention. The invention encompasses methods for treating an autoimmune disease and methods for elimination of cancer cells that express FcγRIIB.
Owner:MACROGENICS INC
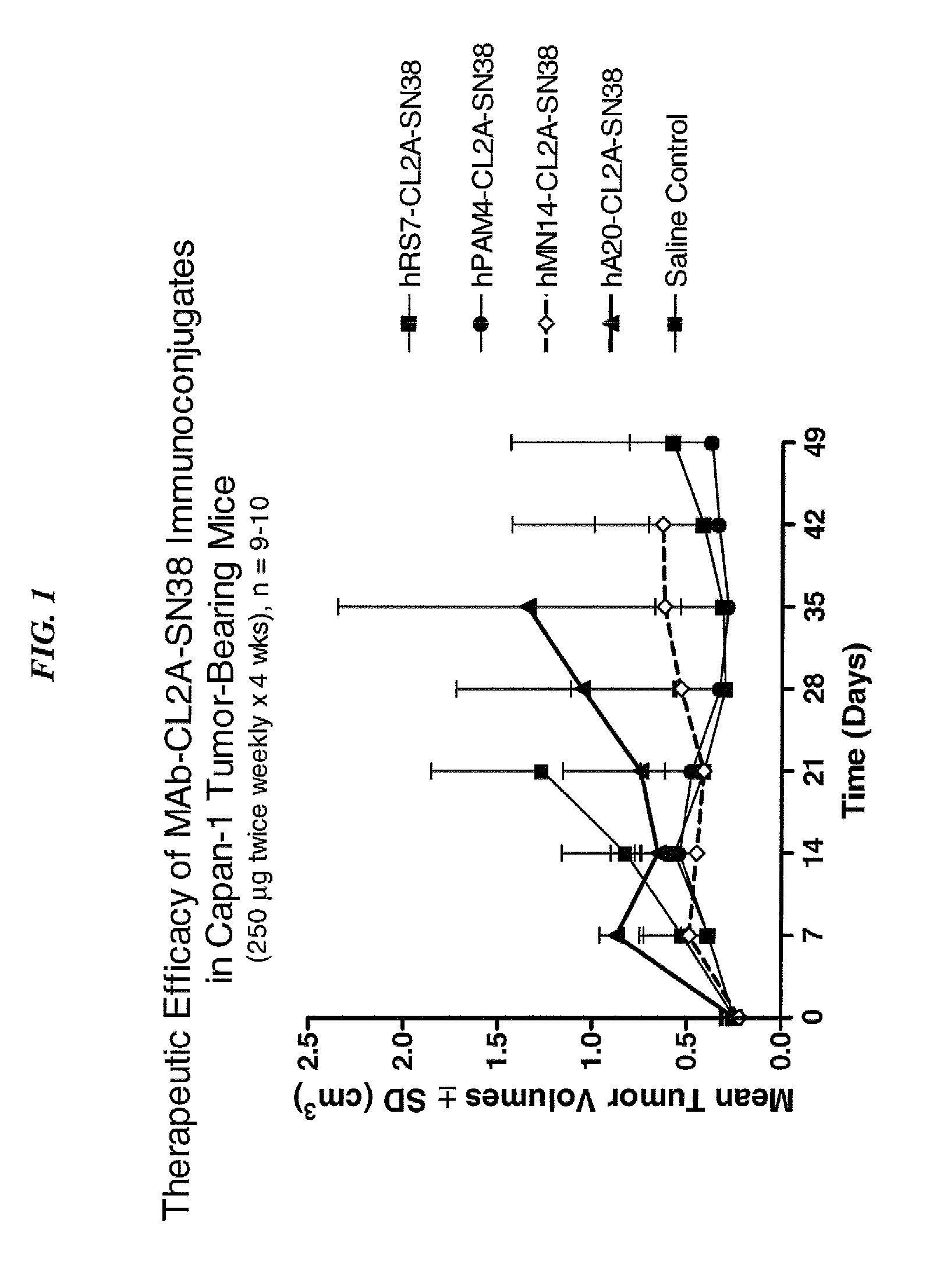
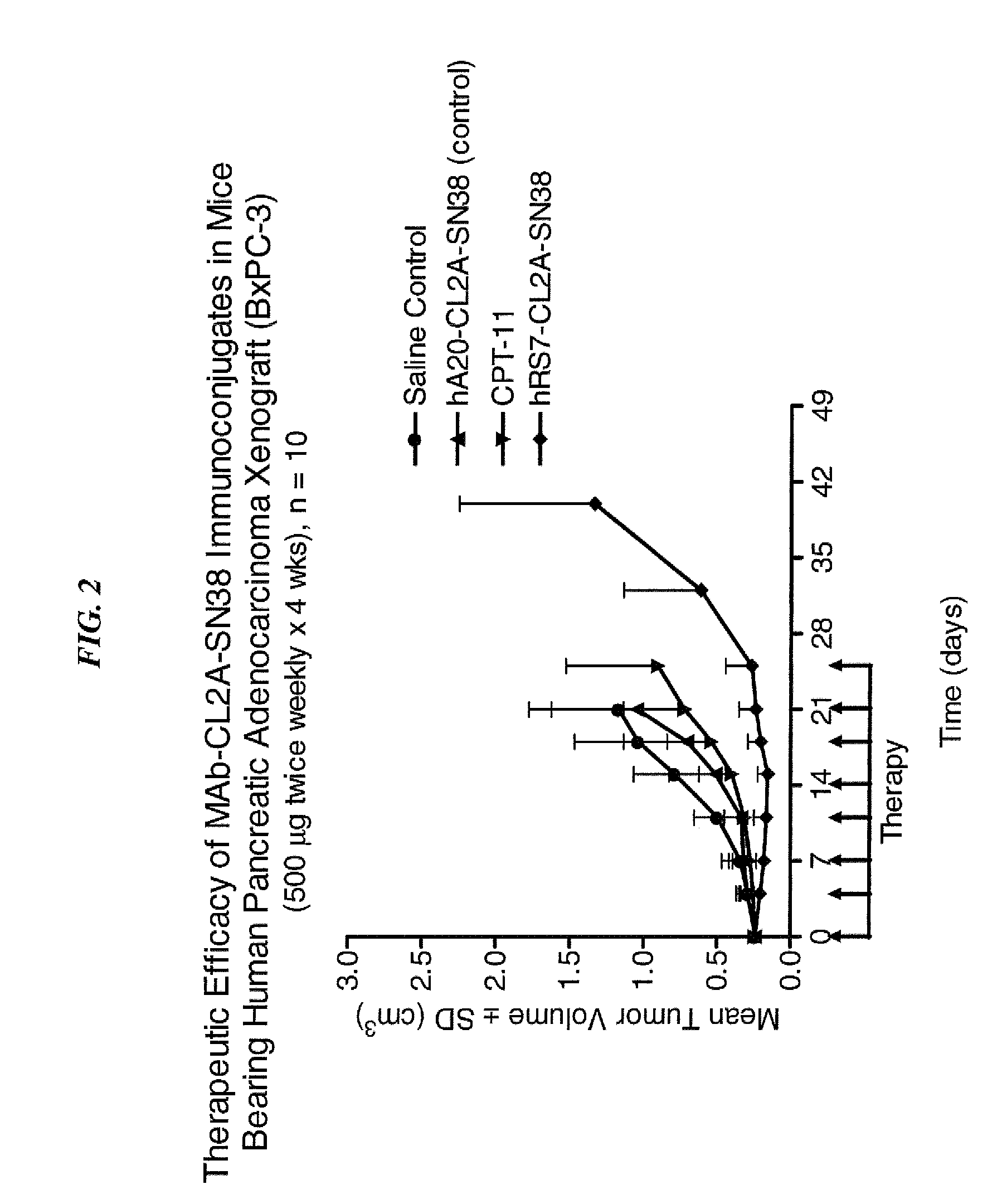
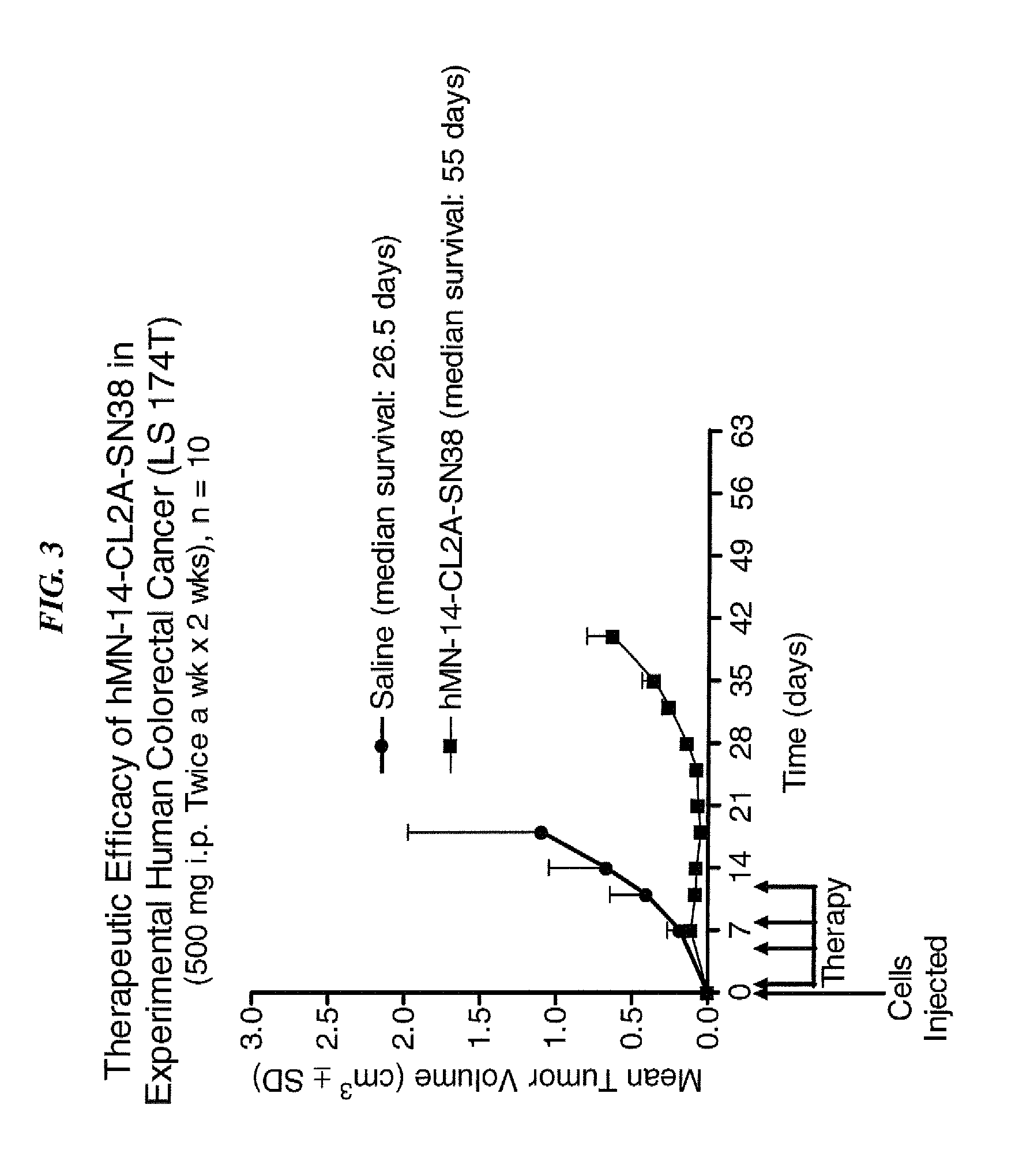
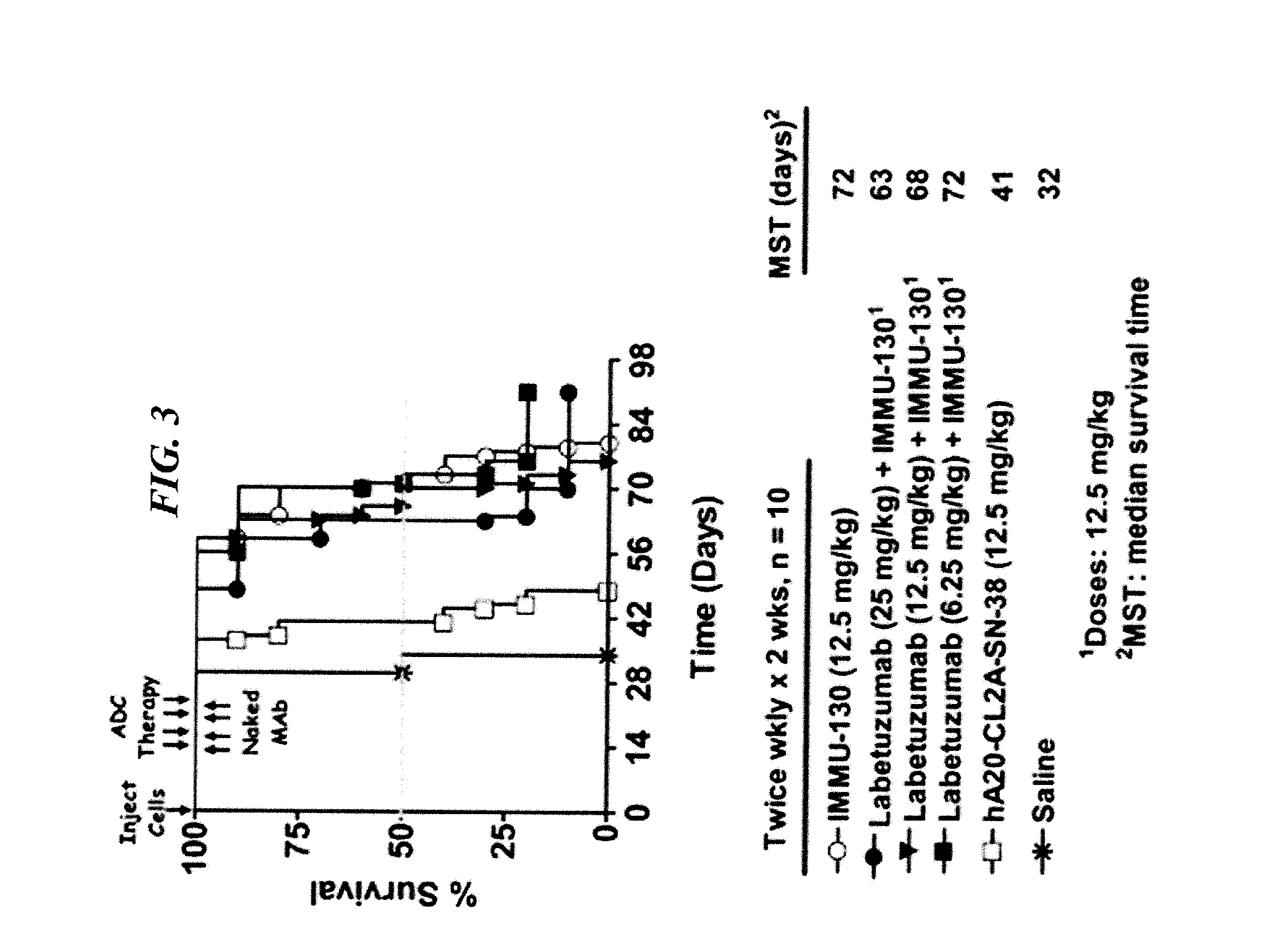
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
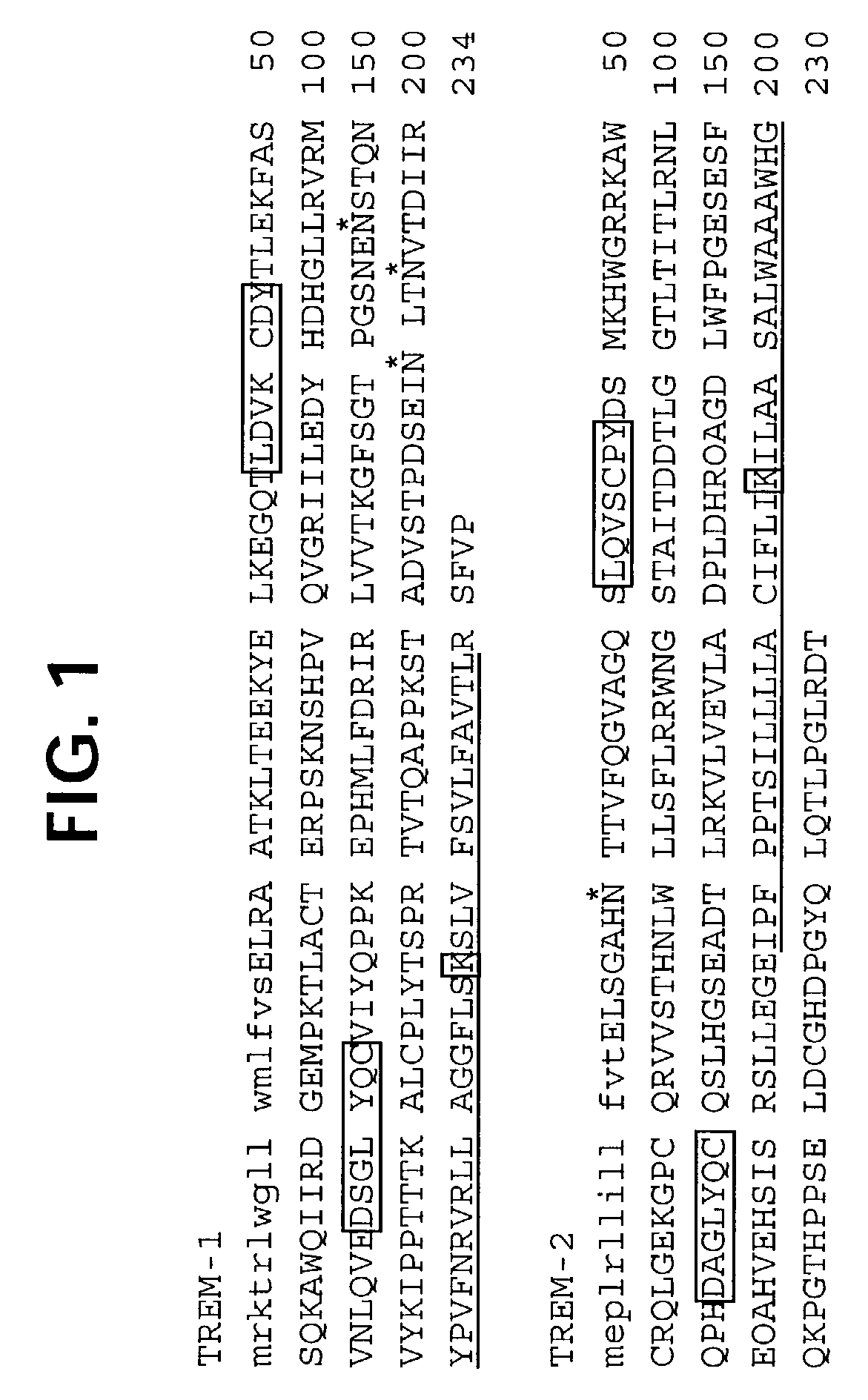
Novel receptor TREM (triggering receptor expressed on myeloid cells) and uses thereof
InactiveUS20030165875A1Strong upregulationAntibacterial agentsAntimycoticsReceptor for activated C kinase 1Monocyte
Novel activating receptors of the Ig super-family expressed on human myeloid cells, called TREM(s) (triggering receptor expressed on myeloid cells) are provided. Specifically, two (2) members of TREMs, TREM-1 and TREM-2 are disclosed. TREM-1 is a transmembrane glycoprotein expressed selectively on blood neutrophils and a subset of monocytes but not on lymphocytes and other cell types and is upregulated by bacterial and fungal products. Use of TREM-1 in treatment and diagnosis of various inflammatory diseases is also provided. TREM-2 is also a transmembrane glycoprotein expressed selectively on mast cells and peripheral dendritic cells (DCs) but not on granulocytes or monocytes. DC stimulation via TREM-2 leads to DC maturation and resistance to apoptosis, and induces strong upregulation of CCR7 and subsequent chemotaxis toward macrophage inflammatory protein 3-beta. TREM-2 has utility in modulating host immune responses in various immune disorders, including autoimmune diseases and allergic disorders.
Owner:BIOXELL
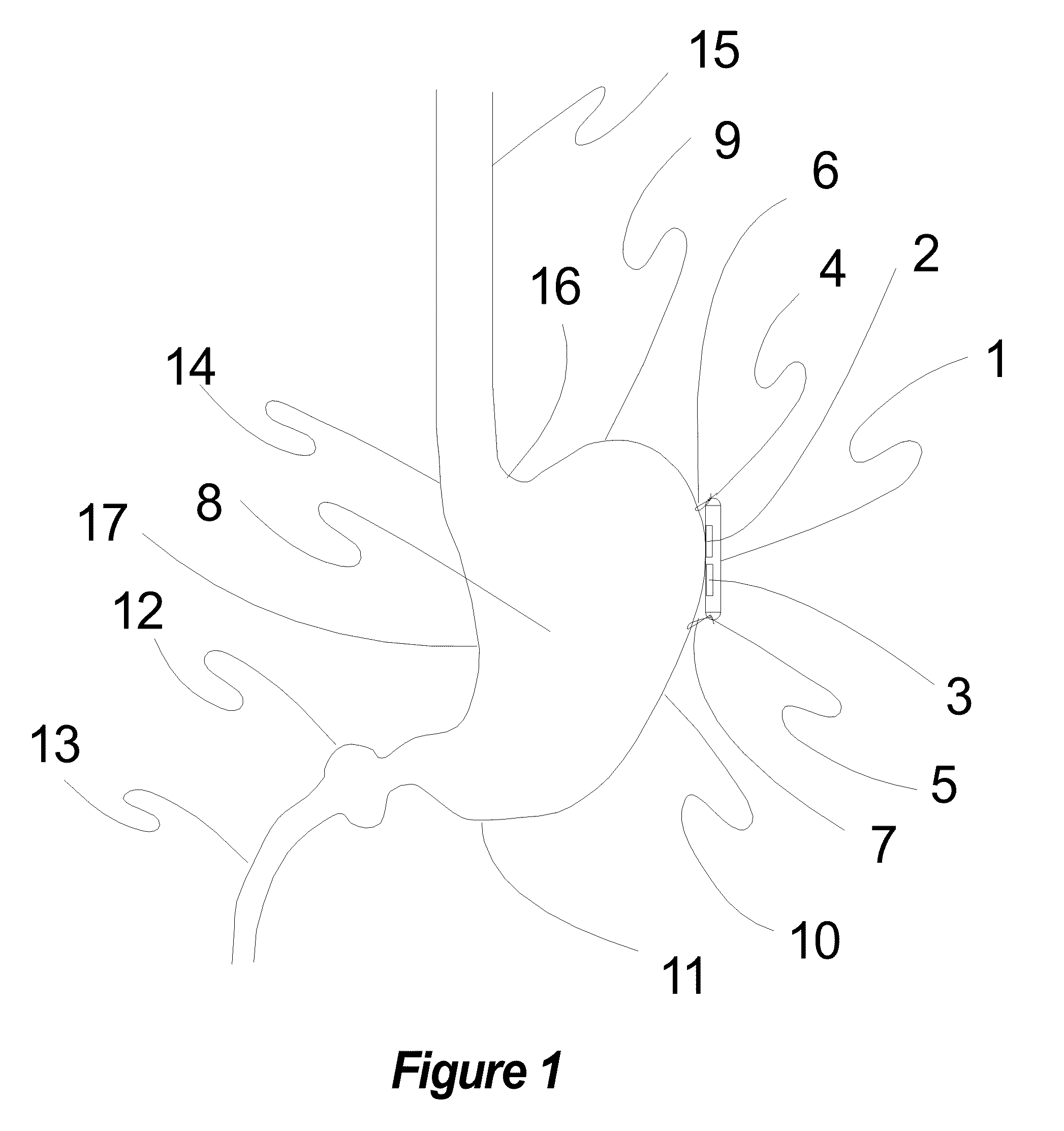
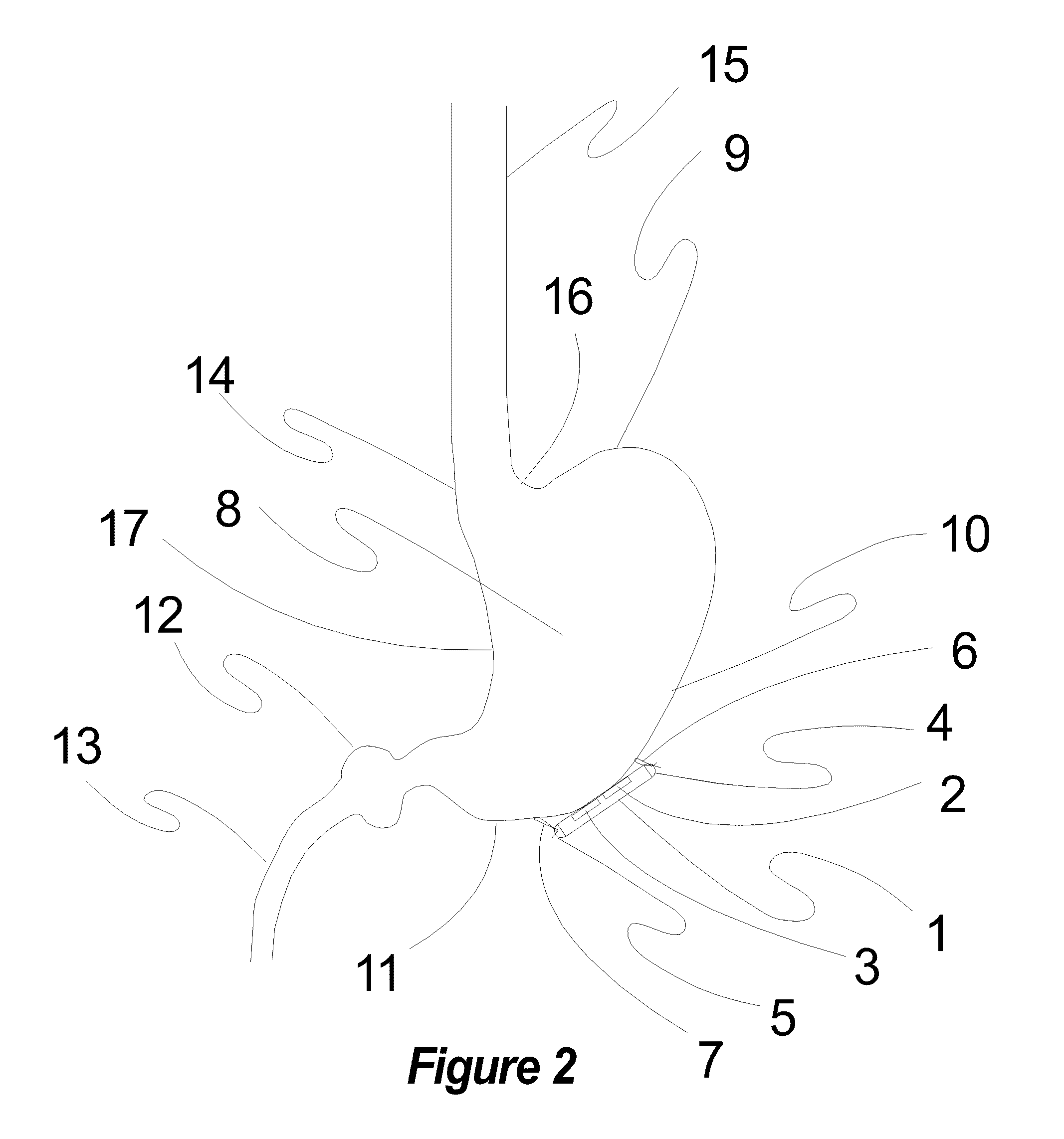
Implantable Neurostimulator with Integral Hermetic Electronic Enclosure, Circuit Substrate, Monolithic Feed-Through, Lead Assembly and Anchoring Mechanism
An implantable medical device is provided for the suppression or prevention of pain, movement disorders, epilepsy, cerebrovascular diseases, autoimmune diseases, sleep disorders, autonomic disorders, abnormal metabolic states, disorders of the muscular system, and neuropsychiatric disorders in a patient. The implantable medical device can be a neurostimulator configured to be implanted on or near a cranial nerve to treat headache or other neurological disorders. One aspect of the implantable medical device is that it includes an electronics enclosure, a substrate integral to the electronics enclosure, and a monolithic feed-through integral to the electronics enclosure and the substrate. In some embodiments, the implantable medical device can include a fixation apparatus for attaching the device to a patient.
Owner:UNITY HA LLC
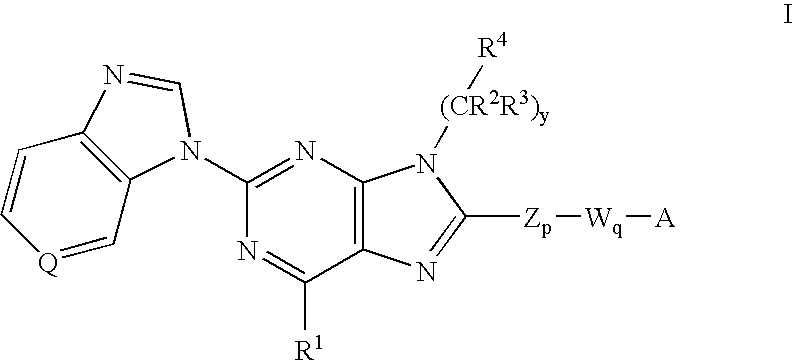
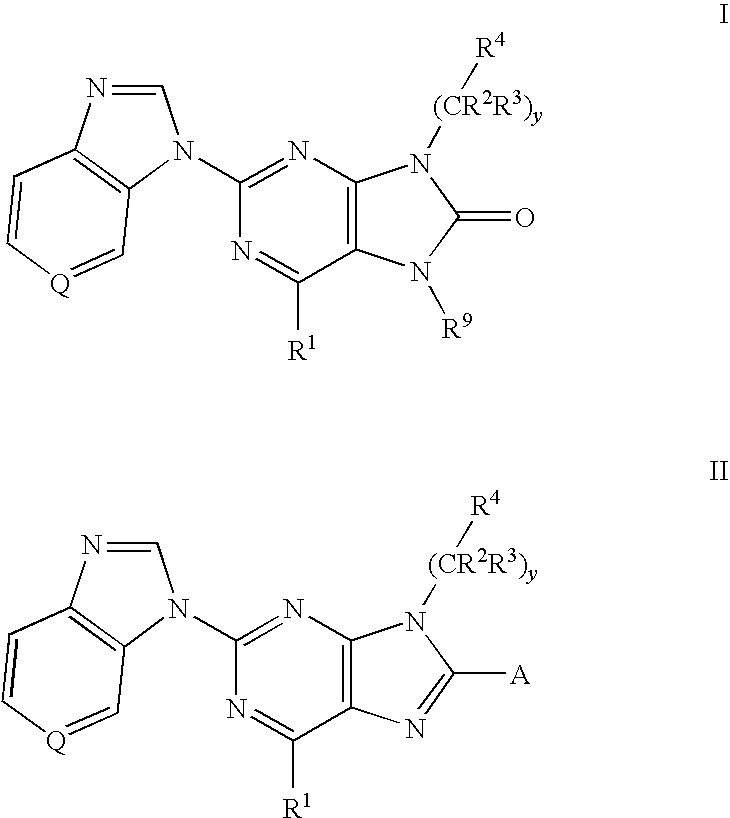
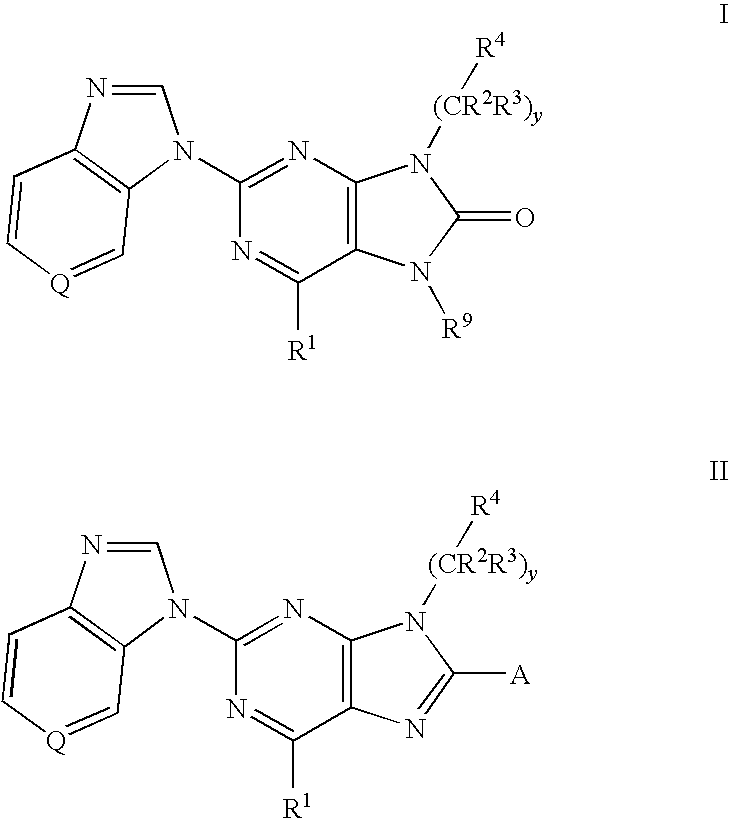
8-substituted 2-(benzimidazolyl)purine derivatives for immunosuppression
The present invention provides novel purines useful for the prevention and treatment of autoimmune diseases, inflammatory disease, mast cell mediated disease and transplant rejection. The compounds are of the general formula I:
Owner:WYETH LLC
Recombinant antibody and antibody fragment
InactiveUS6989145B2Reduce in quantityHigh cytotoxic activitySenses disorderAntibody mimetics/scaffoldsDiseaseDiagnostic agent
A recombinant antibody or the antibody fragment thereof which specifically reacts with an extracellular domain of human CCR4; a DNA which encodes the recombinant antibody or the antibody fragment thereof; a method for producing the recombinant antibody or the antibody fragment thereof; a method for immunologically detecting CCR4, a method for immunologically detecting a cell which expressed CCR4 on the cell surface, a method for depleting a cell which expresses CCR4 on the cell surface, and a method for inhibiting production of Th2 cytokine, which comprise using the recombinant antibody according or antibody fragment thereof; a therapeutic or diagnostic agent for Th2-mediated immune diseases; and a therapeutic or diagnostic agent for a blood cancer.
Owner:KYOWA HAKKO KIRIN CO LTD
Apparatus for autonomic neuromodulation for the treatment of systemic disease
InactiveUS20100241183A1Delay is slowPreserving and prolonging effect of modulationSpinal electrodesUltrasound therapyNervous systemEfferent
A method, apparatus, and surgical technique for the modulation of autonomic function, for the purpose of treating any of several conditions and diseases, including obesity, metabolic disorders, endocrine disorders, diabetes, respiratory disease, asthma, inflammatory disease, immunological disease, infection, cancer, cardiac disease, cardiovascular disease, cerebrovascular disease, stroke, vasospasm, vascular disease, psychiatric disease, depression, affective disorders, anxiety disorders, and other conditions. This includes neural and tissue modulators, including implanted devices, used to modulate efferent and afferent autonomic neurons to influence or control autonomic or other neural function, including modulation of sympathetic and parasympathetic nervous system components as well as their combination.
Owner:DILORENZO BIOMEDICAL
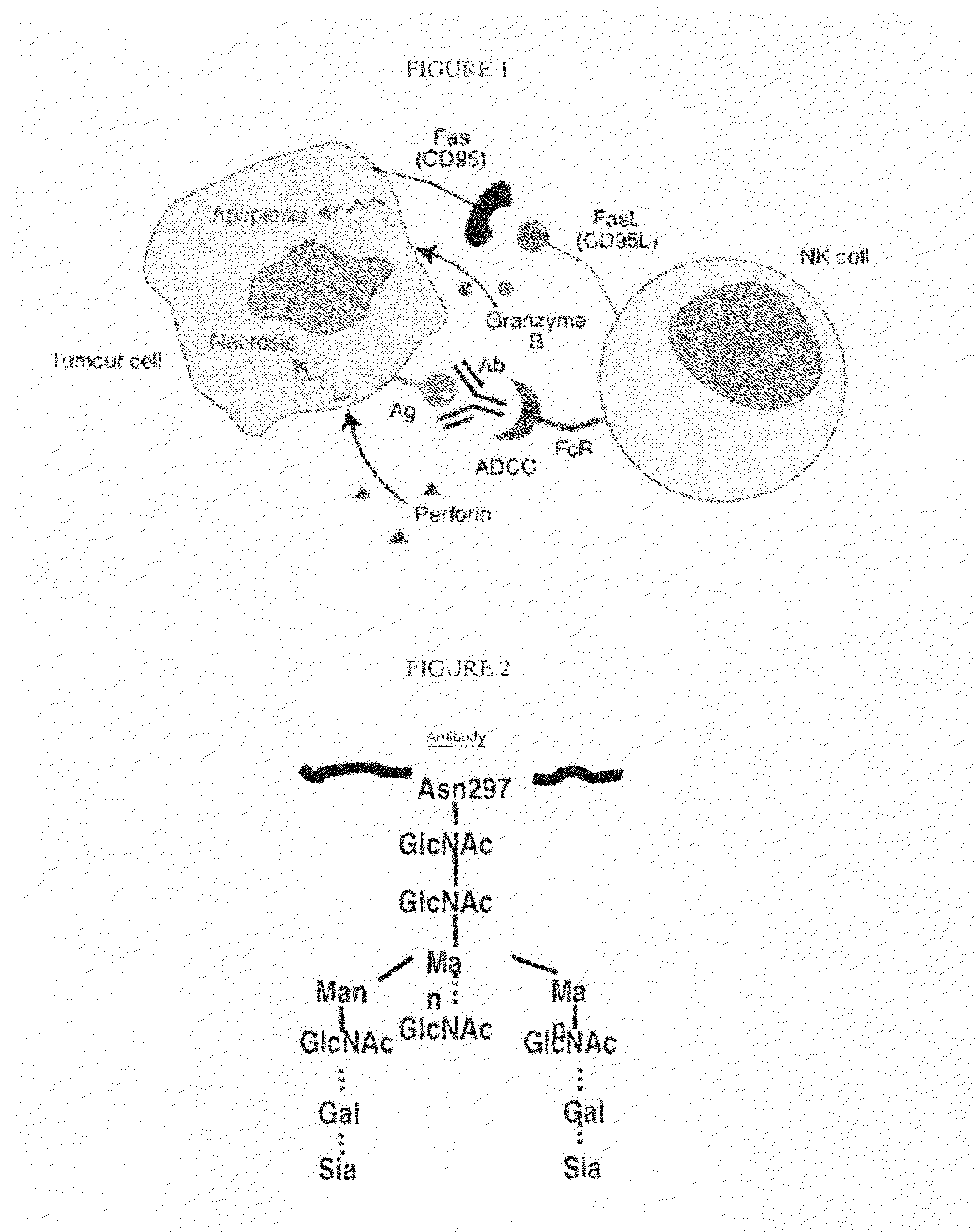
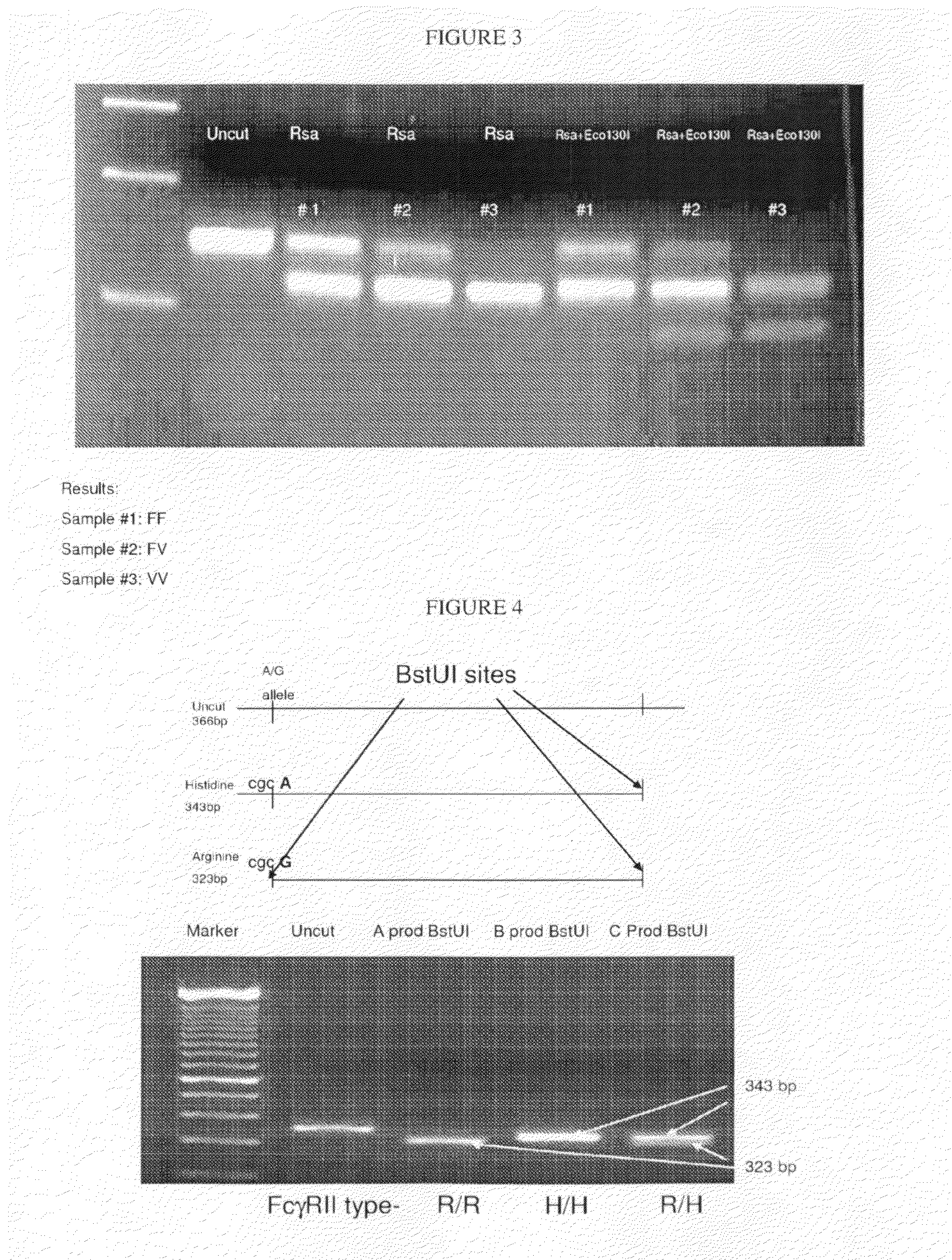
Glycosylation engineered antibody therapy
InactiveUS20100173323A1Good curative effectLow toxicityAntibody ingredientsImmunoglobulinsDiseaseDrug biological activity
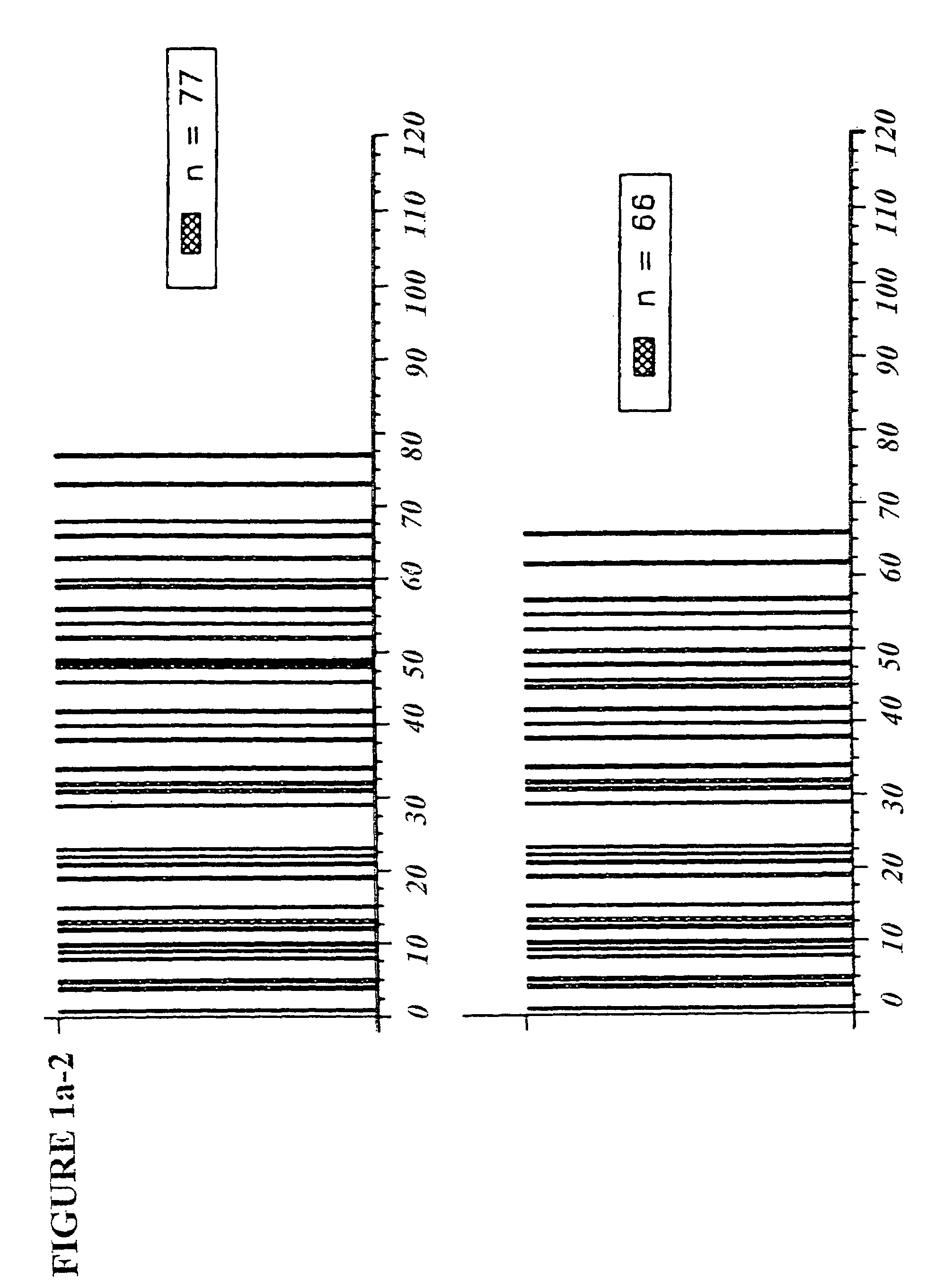
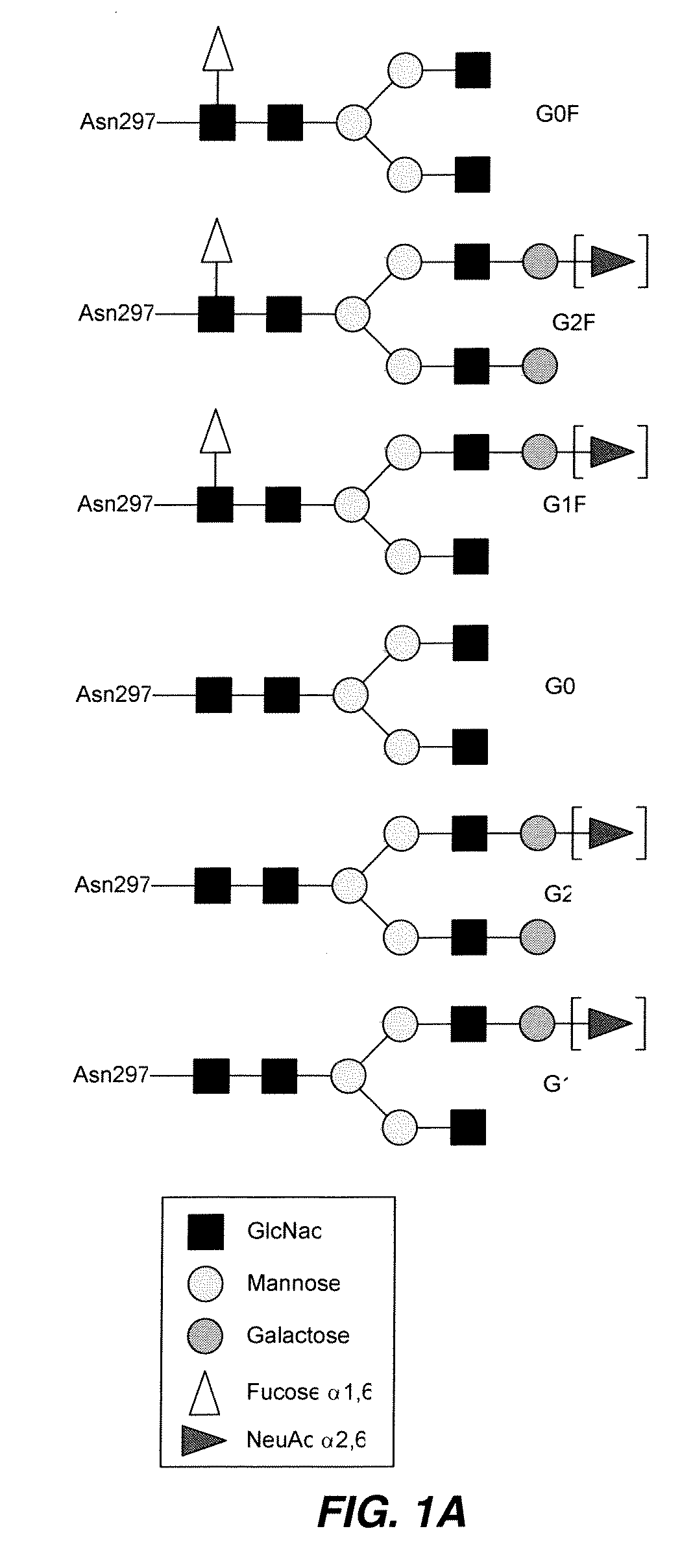
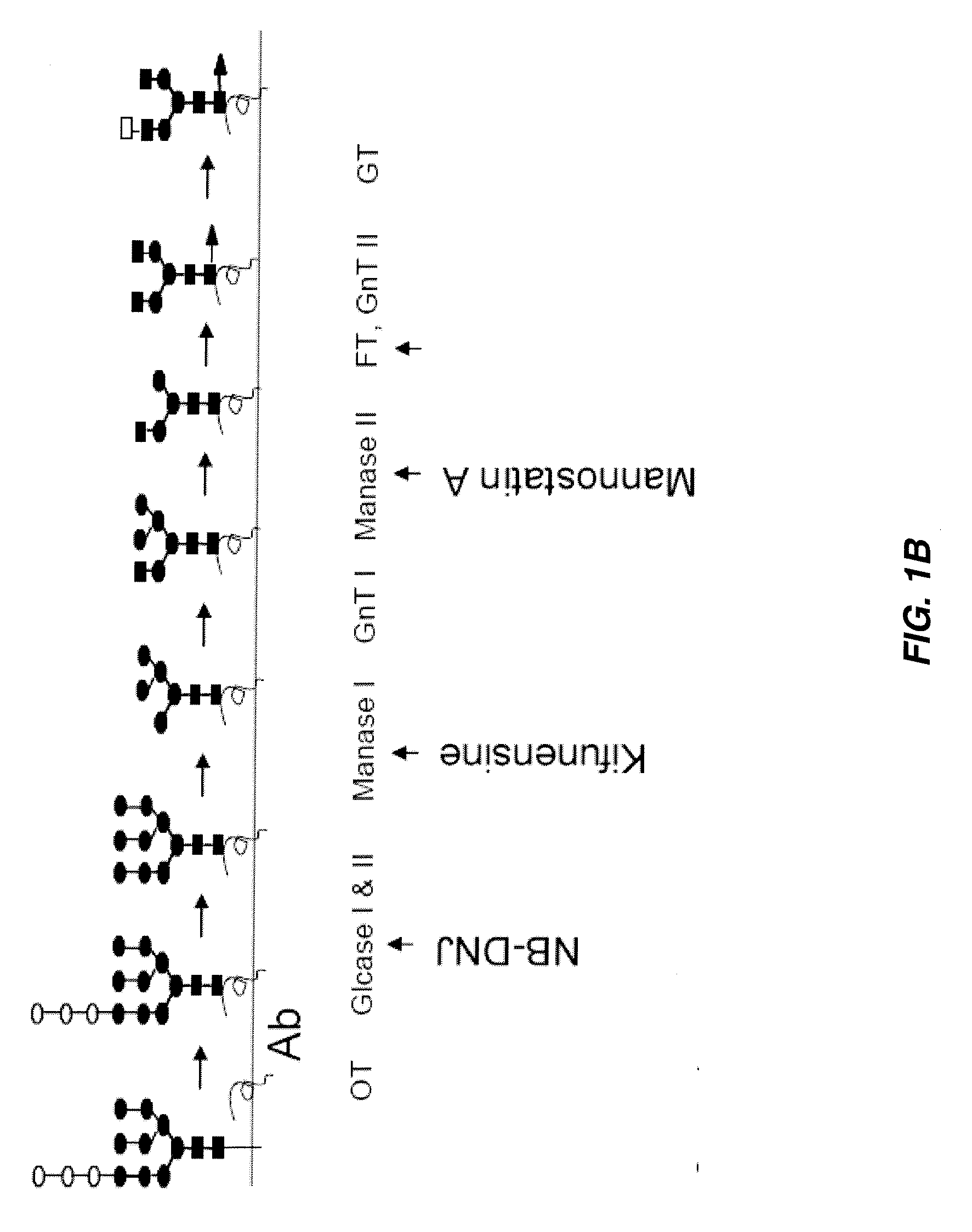
The instant invention is drawn to methods of generating a glycosylation-engineered antibody, and using the glycosylation-engineered antibody for treating a patient, particularly a cancer patient or a patient with an immune disease or disorder. The instant invention is also drawn to methods of generating a glycosylation-engineered antibody for use in the treatment of patients having a polymorphism that does not respond to conventional antibody therapy. The instant invention is also drawn to methods of improving the biological activity of an antibody by glycosylation engineering. The instant invention is also drawn to methods of modulating antibody-dependent cell-mediated cytoxicity (ADCC) using a glycosylation-engineered antibody.
Owner:UNIV OF MARYLAND BIOTECH INST +1
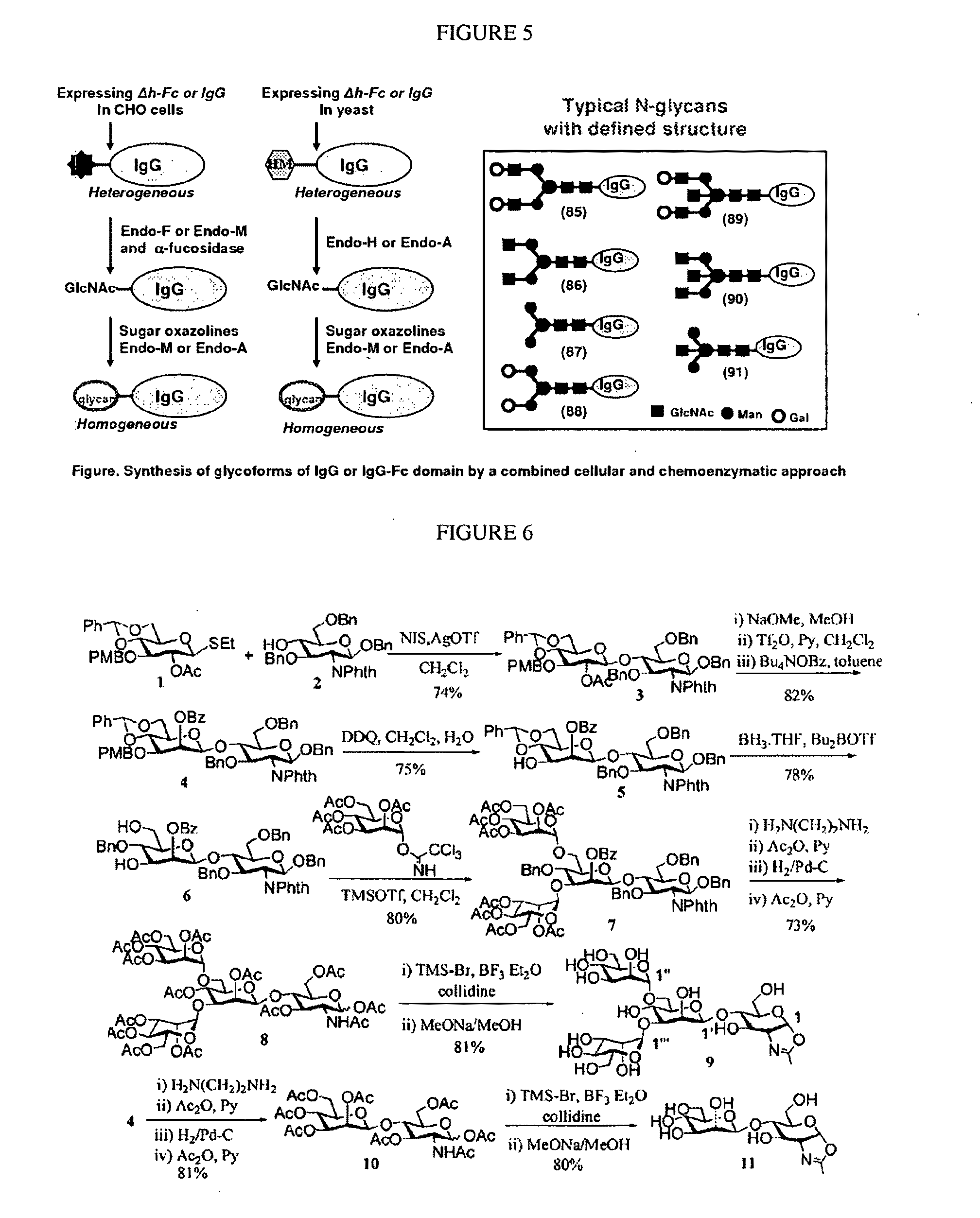
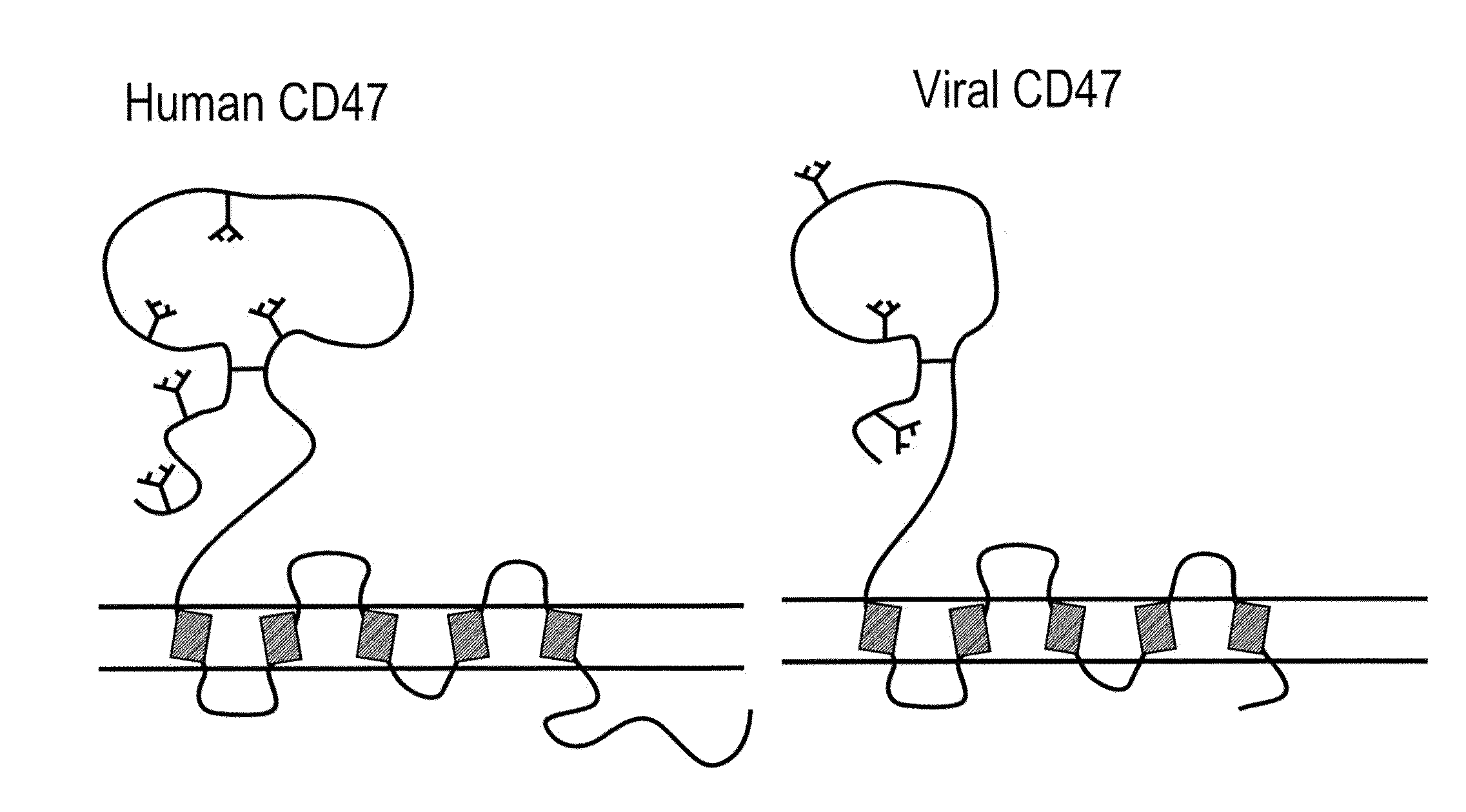
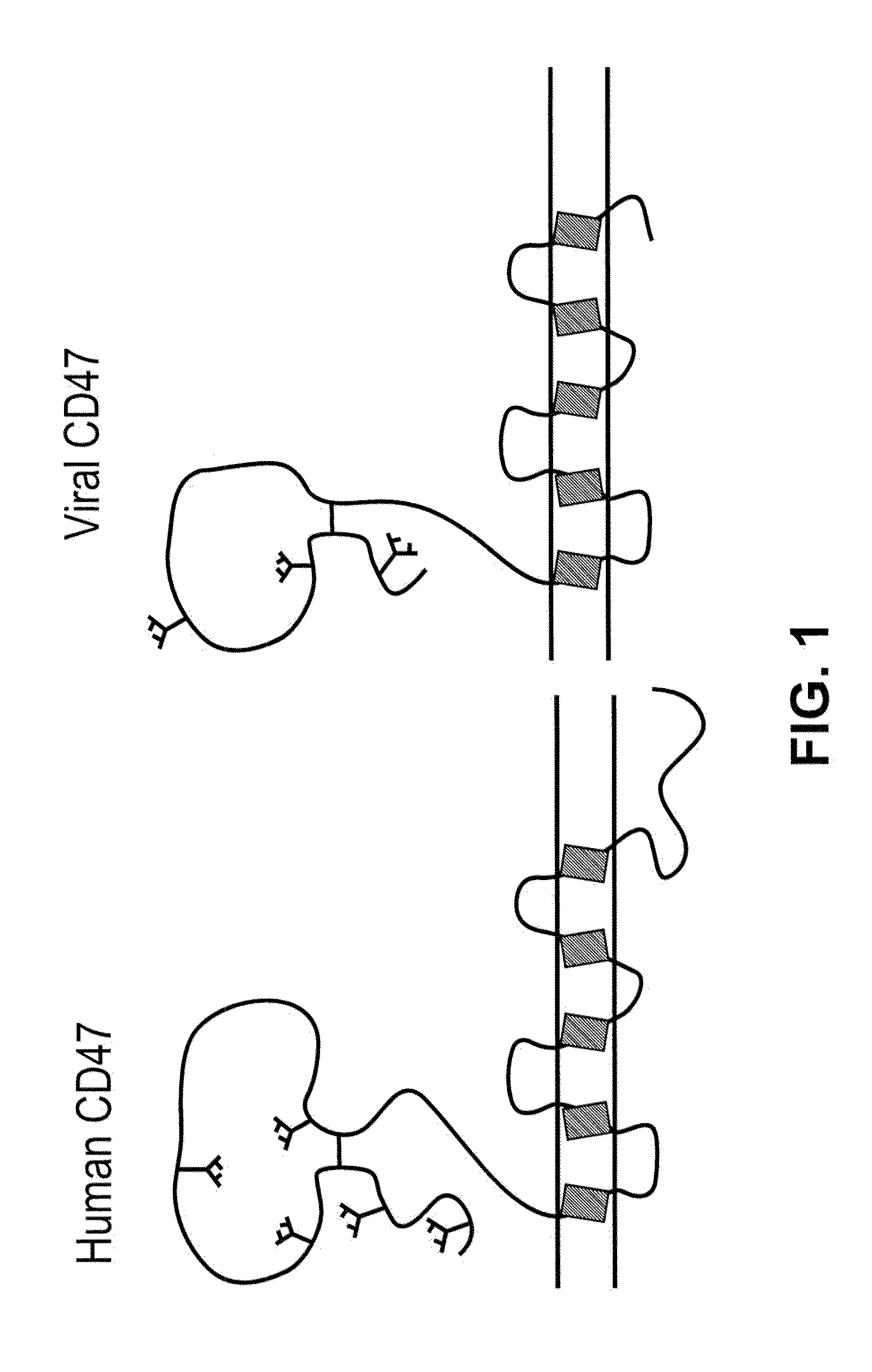
CD47 Related Compositions and Methods for Treating Immunological Diseases and Disorders
Provide herein are fusion polypeptides that comprise a CD47 extracellular domain or a variant thereof that is fused to a Fc polypeptide. The fusion polypeptides are useful for treating an immunological disease or disorder in a subject according to the methods described herein. The fusion polypeptides are capable of suppressing immunoresponsiveness of an immune cell, inhibiting production of proinflammatory cytokines, including inhibiting immune complex-induced production of cytokines.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Copolymer 1 related polypeptides for use as molecular weight markers and for therapeutic use
InactiveUS7074580B2Accurate and robust calibration setTherapeutic utilityPeptide/protein ingredientsDisease diagnosisRetention timeLinear relationship
The present invention provides processes for determining the molecular weight of glatiramer acetate and other copolymers using molecular weight markers. The present invention further provides a plurality of molecular weight markers for determining the molecular weight of glatiramer acetate and other copolymers which display linear relationships between molar ellipticity and molecular weight, and between retention time and the log of the molecular weight. The molecular weight markers also optimally demonstrate biological activity similar to glatiramer acetate or corresponding copolymers and can be used for treating or preventing various immune diseases.
Owner:YEDA RES & DEV CO LTD
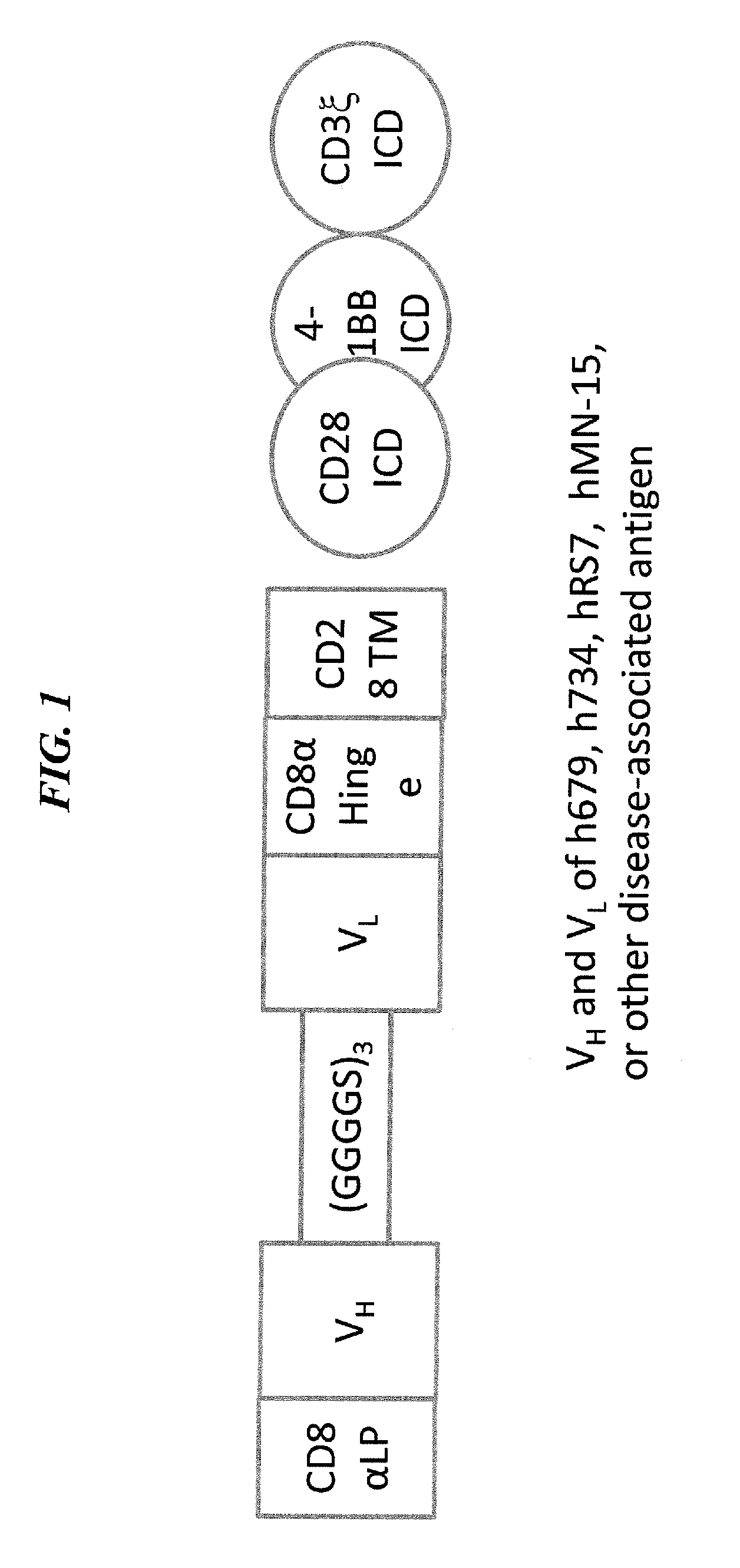
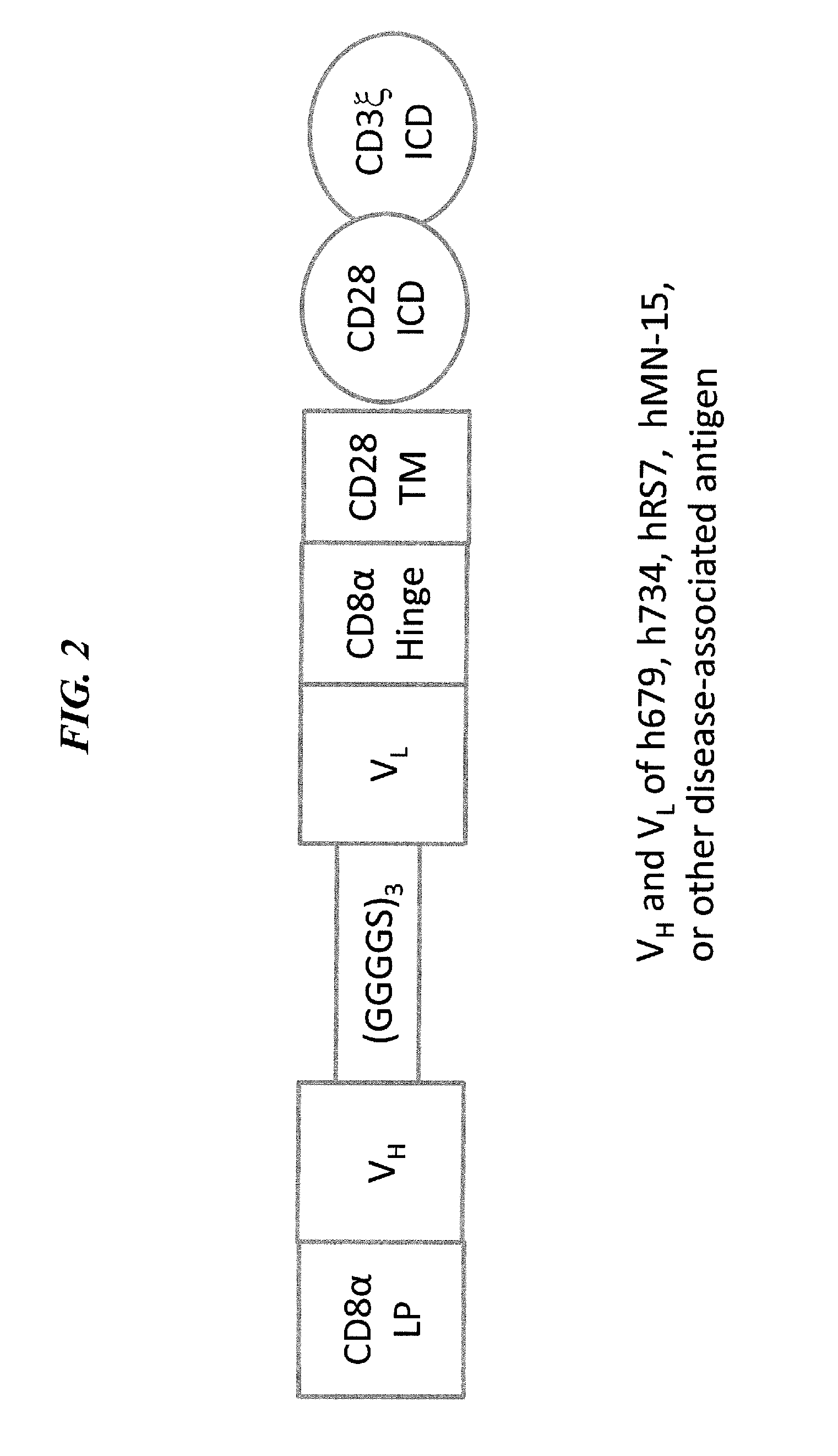
Disease therapy with chimeric antigen receptor (CAR) constructs and t cells (car-t) or nk cells (car-nk) expressing car constructs
InactiveUS20160361360A1Maintain self-toleranceModulate durationAntibacterial agentsPeptide/protein ingredientsAutoimmune conditionDebulking Procedure
The present invention concerns CAR, CAR-T and CAR-NK constructs, preferably comprising a scFv antibody fragment against a disease-associated antigen or a hapten. More preferably, the antigen is a TAA, such as Trop-2. The constructs may be administered to a subject with a disease, such as cancer, autoimmune disease, or immune dysfunction disease, to induce an immune response against disease-associated cells. Where the constructs bind to a hapten, the subject is first treated with a hapten-conjugated antibody that binds to a disease associated antigen. Therapy may be supplemented by other treatments, such as debulking procedures (e.g., surgery, chemotherapy, radiation therapy) or coadministration of other agents. More preferably, administration of the construct is preceded by predosing with an unconjugated antibody that binds to the same disease-associated antigen. Most preferably, an antibody against CD74 or HLA-DR is administered to reduce systemic immunotoxicity induced by the constructs.
Owner:IMMUNOMEDICS INC
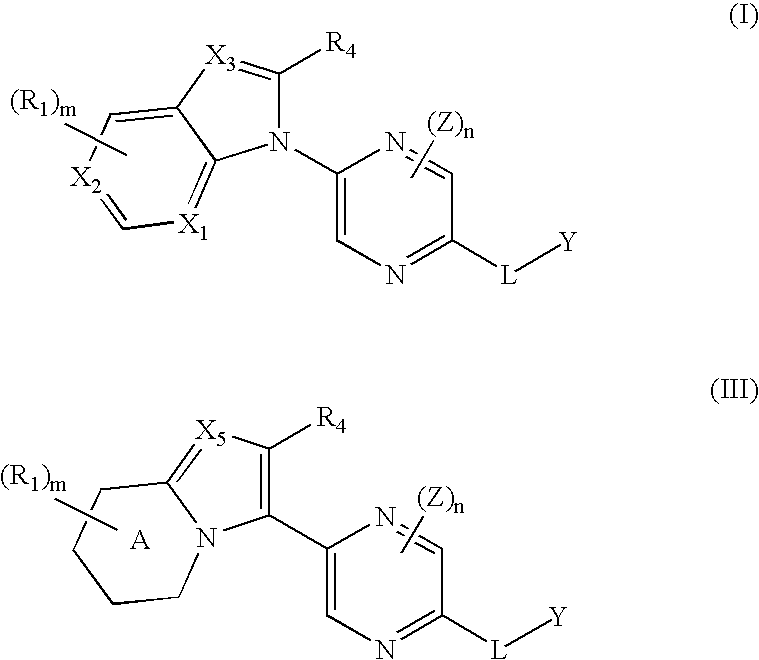
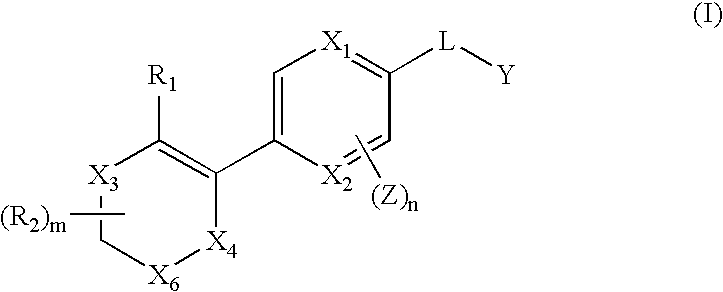
Benzoimidazolyl-pyrazine compounds for inflammation and immune-related uses
The invention relates to compounds of structural formula (I) or (III): or a pharmaceutically acceptable salt, solvate, clathrate, or prodrug thereof, wherein ring A, X1, X2, X3, X5, R1, R4, Y, Z, L, m and n are defined herein. These compounds are useful as immunosuppressive agents and for treating and preventing inflammatory conditions, allergic disorders, and immune disorders.
Owner:SYNTA PHARMA CORP
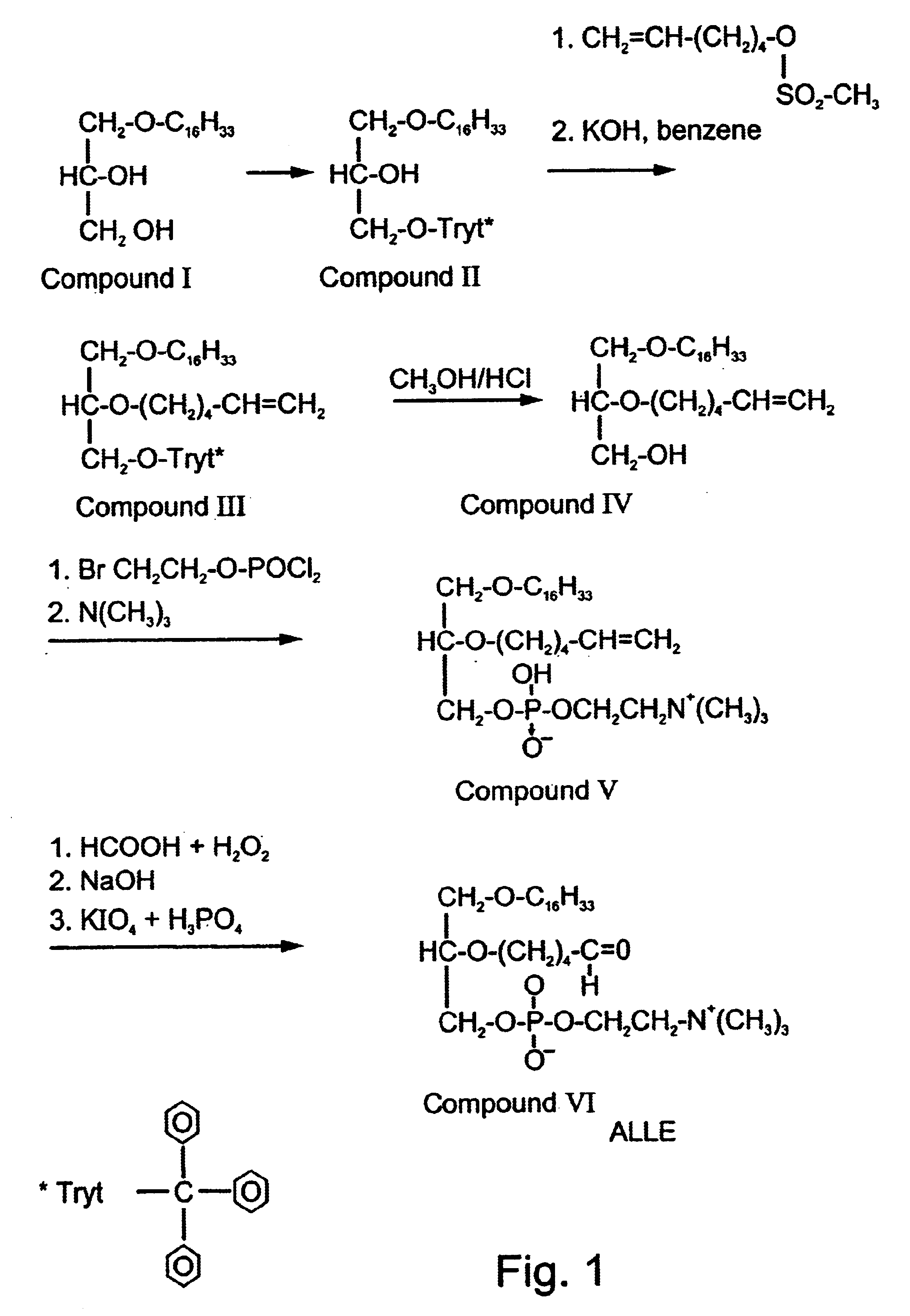
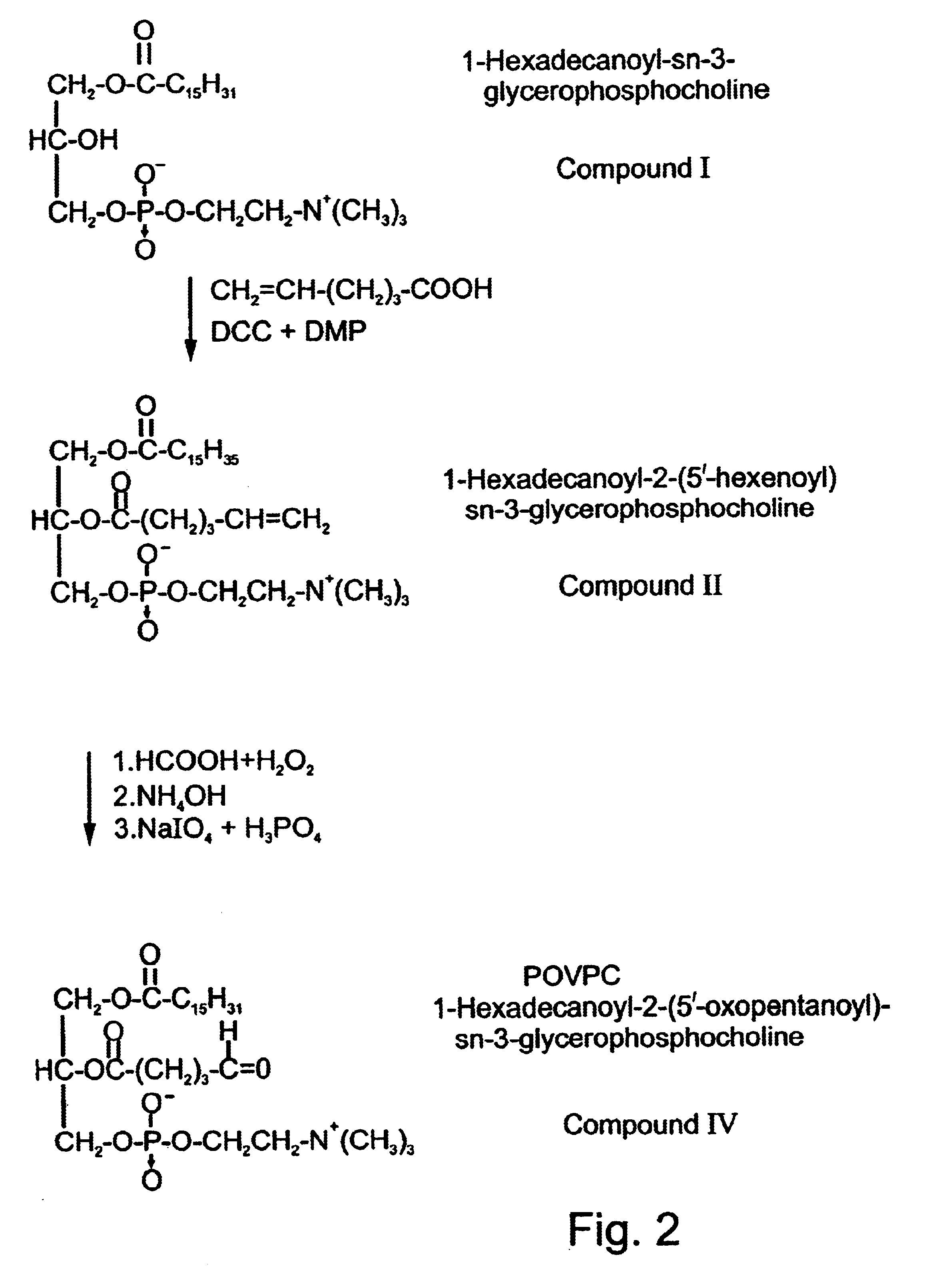
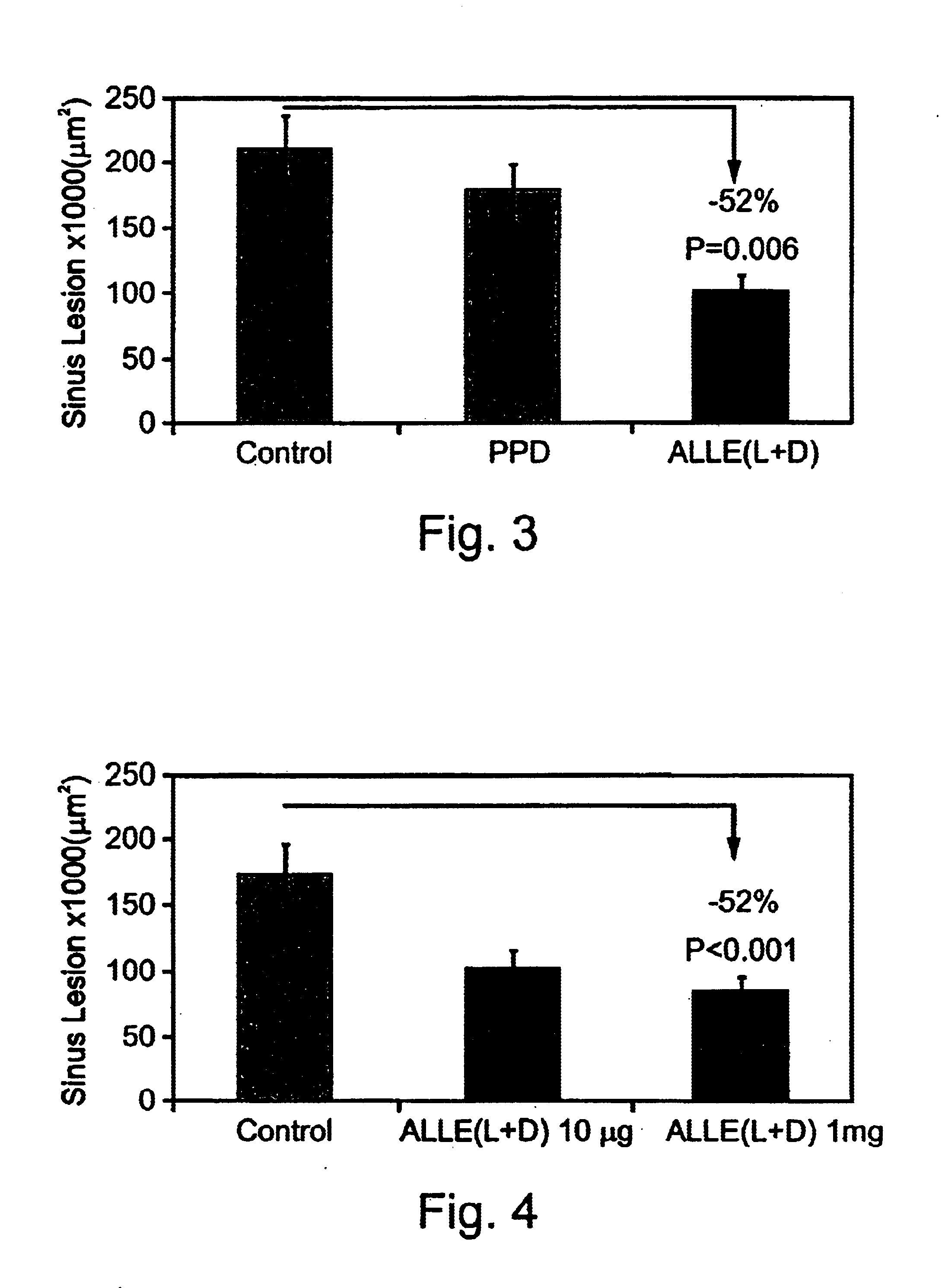
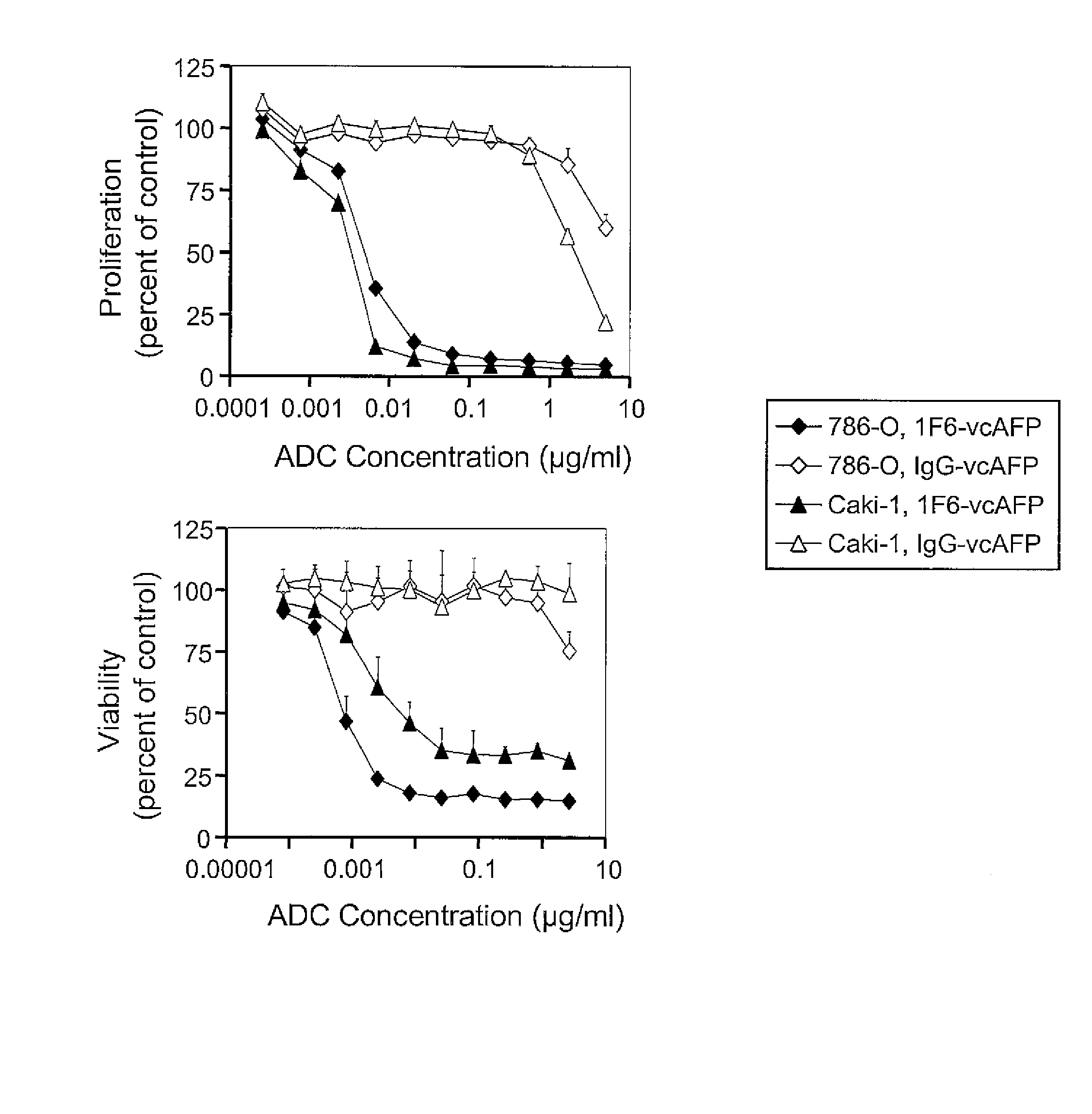
Methods employing and compositions containing defined oxidized phospholipids for prevention and treatment of atherosclerosis
InactiveUS6838452B2Reduce reactivityAntibacterial agentsOrganic active ingredientsMedicineAutoimmune disease
Owner:VASCULAR BIOGENICS
Anti-cd70 antibody-drug conjugates and their use for the treatment of cancer and immune disorders
InactiveUS20080025989A1Prevent relapseCompounds screening/testingAntibody ingredientsCytotoxicityAnti-CEA Antibody
Disclosed are anti-CD70 antibodies and derivatives thereof conjugated to cytotoxic, immunosuppressive, or other therapeutic agents, as well as pharmaceutical compositions and kits comprising the antibody- and antibody derivative-drug conjugates. Also disclosed are methods, for the treatment of CD70-expressing cancers and immunological disorders, comprising administering to a subject the disclosed pharmaceutical compositions.
Owner:SEATTLE GENETICS INC
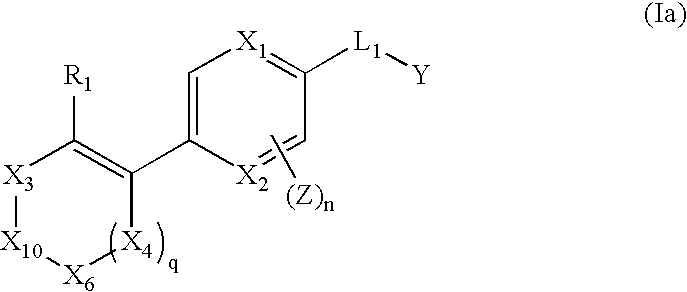
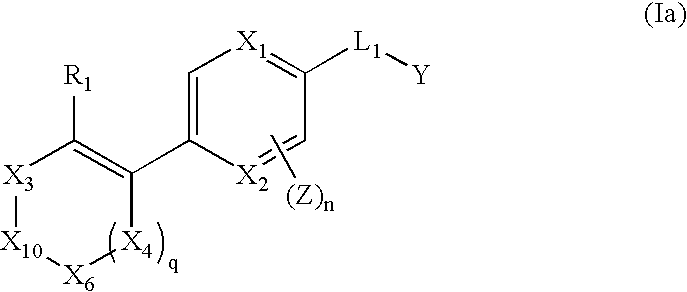
Vinyl-phenyl derivatives for inflammation and immune-related uses
The invention relates to compounds of structural formula (Ia): or a pharmaceutically acceptable salt, solvate, clathrate, or prodrug thereof, wherein X1, X2, X3, X4, X6, X10, R1, Y, Z, L, and n are defined herein. These compounds are useful as immunosuppressive agents and for treating and preventing inflammatory conditions, allergic disorders, and immune disorders.
Owner:SYNTA PHARMA CORP
Method of diagnosing autoimmune disease
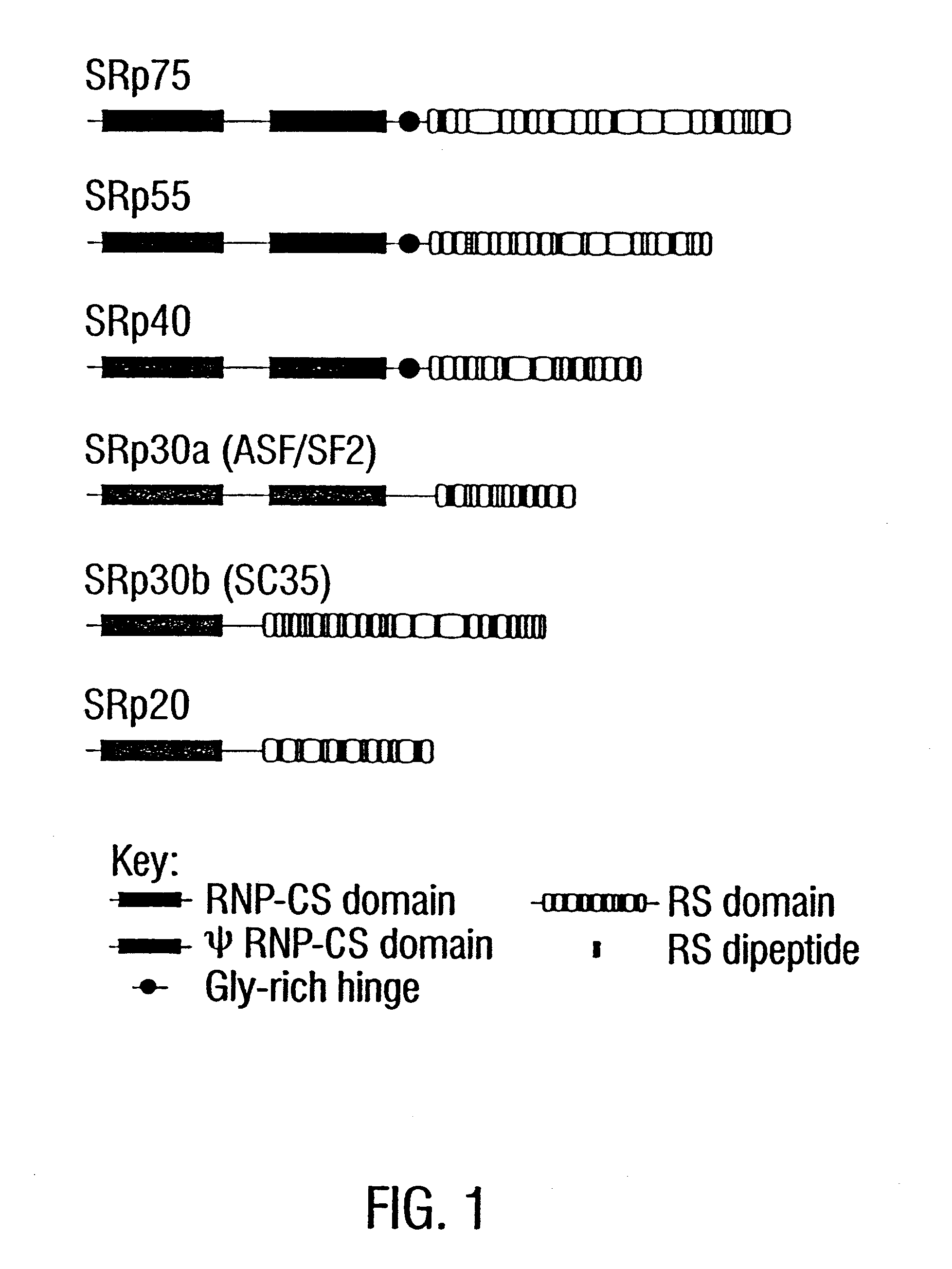
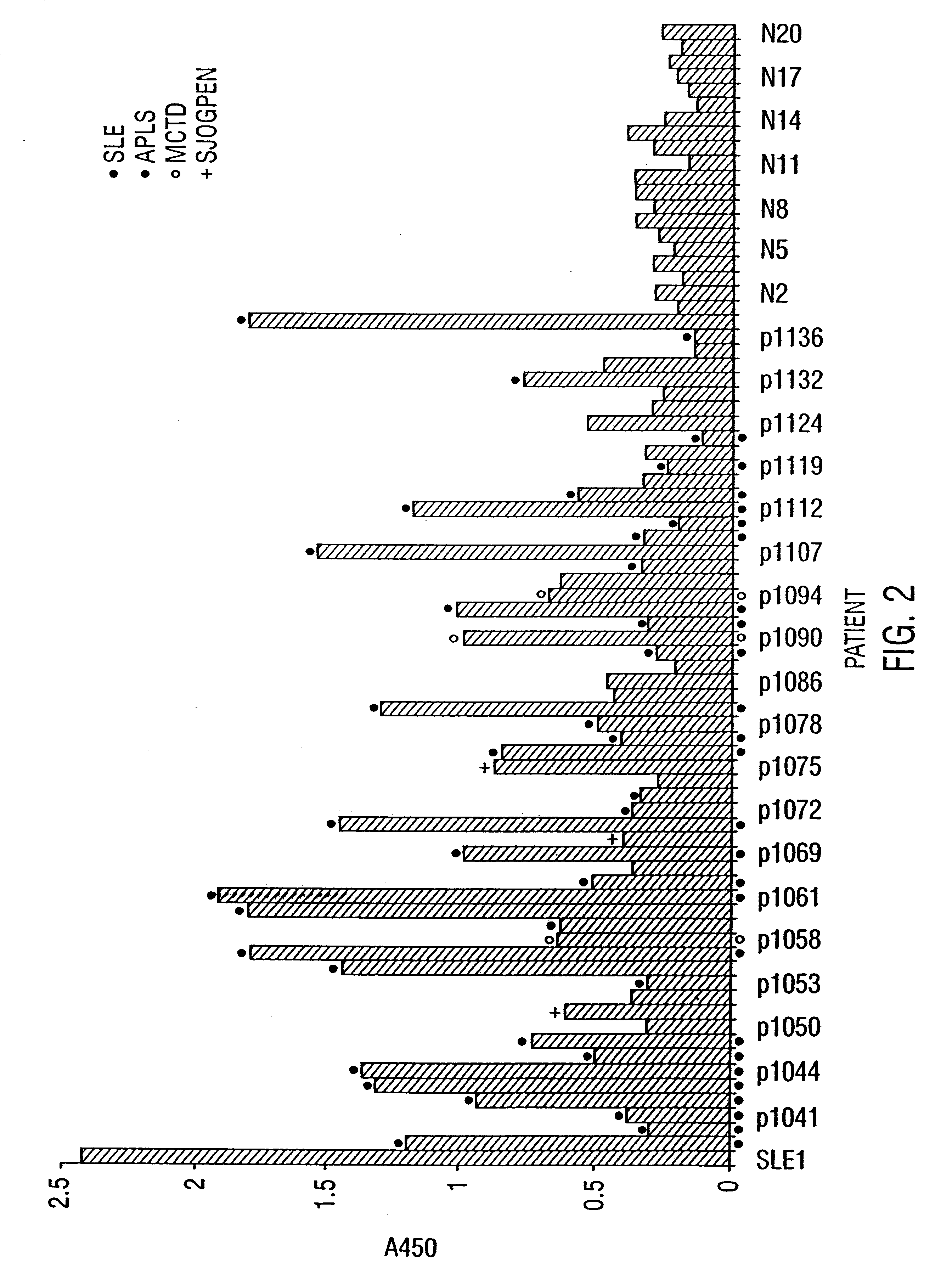
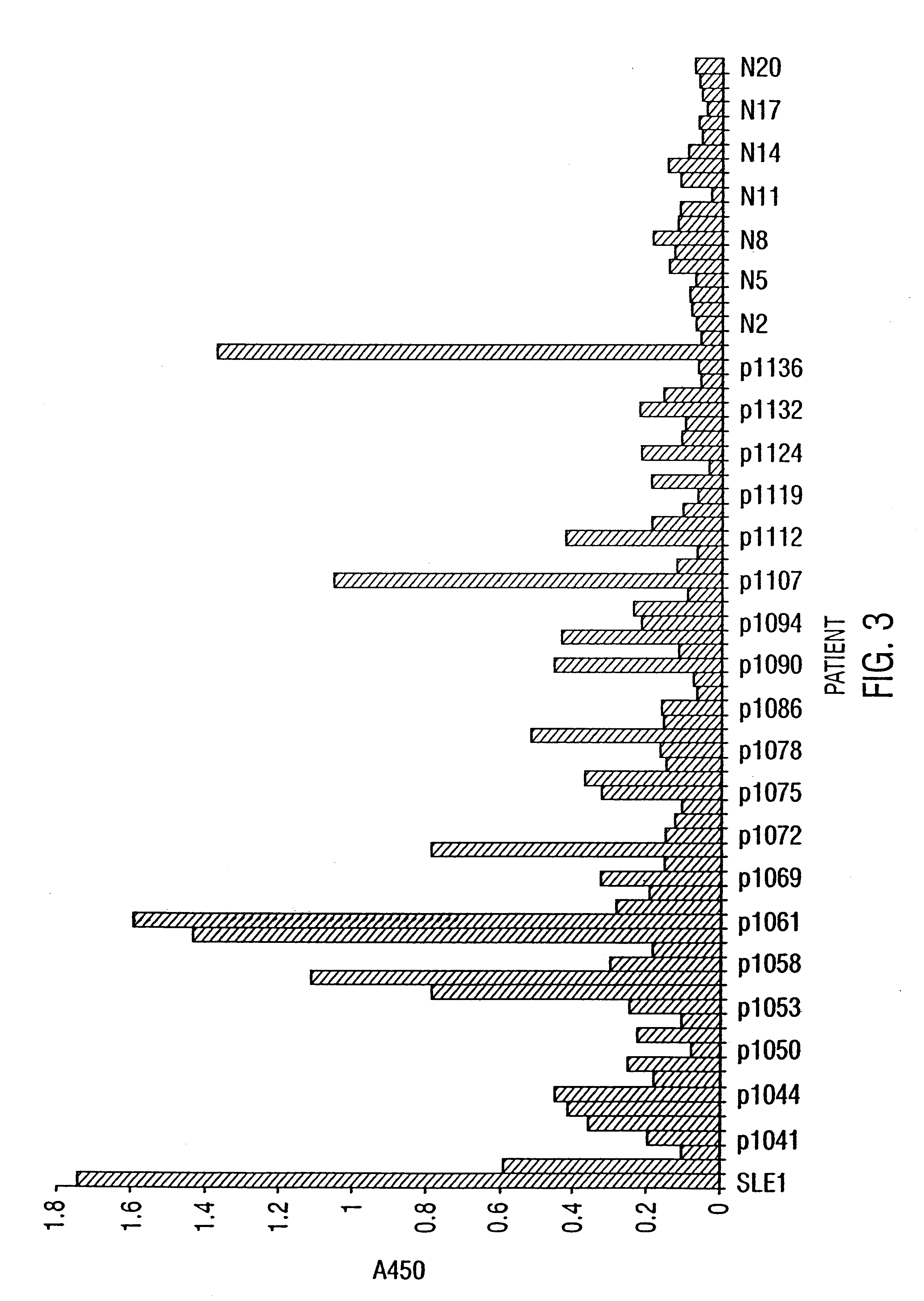
The present invention relates to diagnostic applications. For autoimmune diseases more particularly, it is demonstrated herein that individuals with SLE, APLA, MCDS and PSS have antibodies that are specific for SR proteins. Thus, in particular aspects the present invention provides methods and compositions for diagnosing autoimmune disease using SR proteins and antibodies to detect the presence of SR protein-specific antibodies in an individual suspected of having autoimmune disease, wherein the presence of such antibodies is indicative of said individual suffering from autoimmune disease.
Owner:FRED HUTCHINSON CANCER RES CENT
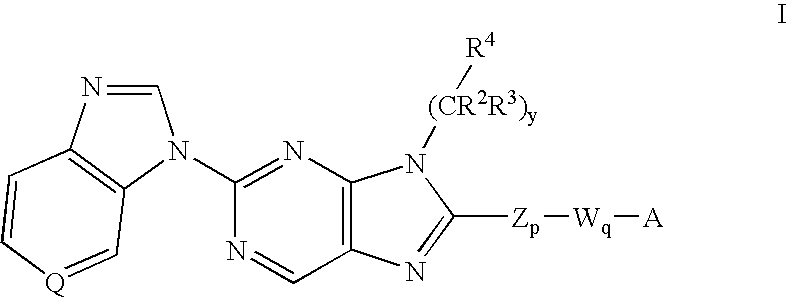
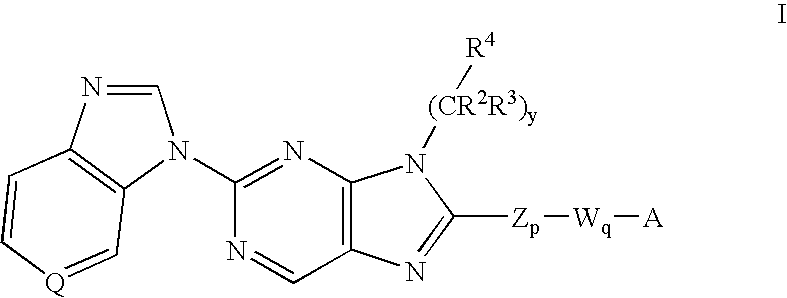
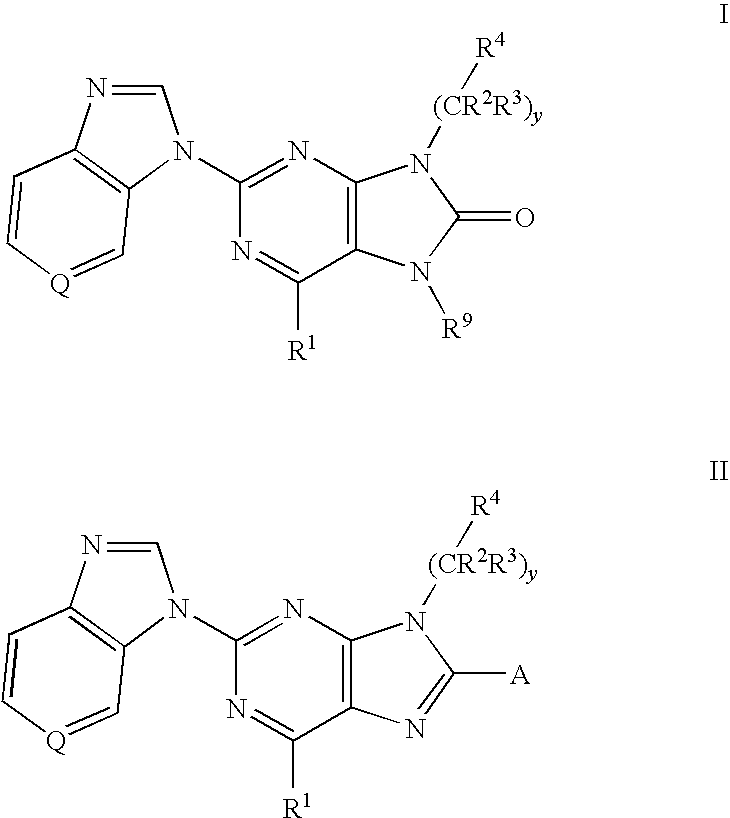
6-substituted 2-(benzimidazolyl)purine and purinone derivatives for immunosuppression
The present invention provides novel purinones and purines useful for the prevention and treatment of autoimmune diseases, inflammatory disease, mast cell mediated disease and transplant rejection. The compounds are of the general formulae I and II:
Owner:WYETH LLC
Thiazole and thiadiazole compounds for inflammation and immune-related uses
InactiveUS20070254925A1Suppress immune systemInhibit activationBiocideSenses disorderHypersensitivity DiseasesImmune disorder
The invention relates to compounds of structural formula (I): or a pharmaceutically acceptable salt, solvate, clathrate, or prodrug thereof, wherein R′1, X, X′, L and Y are defined herein. These compounds are useful as immunosuppressive agents and for treating and preventing inflammatory conditions, allergic disorders, and immune disorders.
Owner:SYNTA PHARMA
Copolymer 1 related polypeptides for use as molecular weight markers and for therapeutic use
InactiveUS7163802B2Accurate and robust calibration setTherapeutic utilityPeptide/protein ingredientsDisease diagnosisRetention timeLinear relationship
The present invention provides processes for determining the molecular weight of glatiramer acetate and other copolymers. The present invention further provides a plurality of molecular weight markers for determining the molecular weight of glatiramer acetate and other copolypmers which display linear relationships between molar ellipticity and molecular weight, and between retention time and the log of the molecular weight. The molecular weight markers also optimally demonstrate biological activity similar to glatiramer acetate or corresponding copolymers and can be used for treating or preventing various immune diseases. In addition, the subject invention provides pharmaceutical compositions for the treatment of immune diseases comprising a polypeptide having an identified molecular weight and an amino acid composition corresponding to glatiramer acetate or a terpolymer.
Owner:YEDA RES & DEV CO LTD
Antibody-based therapeutics with enhanced ADCC activity
Methods for producing antibody-based therapeutics with enhanced ADCC activity are disclosed. The enhanced ADCC activity is attributed to oligomannose-type N-glycans on the antibodies and Fc fusion proteins of the invention. Also disclosed are methods of using such antibody-based therapeutics for targeted killing of cells in a mammal, including therapeutic methods of treating cancers, autoimmune diseases and other diseases.
Owner:GENZYME CORP
Methods and compositions for inducing oral tolerance in mammals
The present invention relates to methods and pharmaceutical formulations for orally delivering an antigen to induce tolerance. The antigen is combined with derivatized amino acids or salts thereof. The induction of oral tolerance may be applied clinically for the prevention or treatment of auto-immune diseases and clinical allergic hypersensitivities, and for the prevention of allograft rejection.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com