A method for testing the dissolution profile of immediate-release dosage form drug amoxicillin-clavulanate potassium dispersible tablet
A technology for amoxicillin and clavulanate potassium and dispersible tablets, which is applied in the field of analytical chemistry, and can solve the problem of in vitro release that cannot reflect the in vitro release behavior of amoxicillin and clavulanate potassium dispersible tablets, prescriptions or processes. Regional distinction and other issues to achieve the effect of achieving consistency and ensuring the quality of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] a) Dissolution conditions
[0037] Dissolution tester: RCZ-8M, sampling collection system RZD-8D
[0038] Dissolution medium: medium A, purified water; medium B, pH 1.2 solution; medium C, pH 4.0 acetic acid / sodium acetate buffer; medium D, pH 6.8 phosphate buffer. 900ml each.
[0039] Medium preparation, medium B, take 2.0g of sodium chloride, add appropriate amount of water to dissolve, add 7ml of hydrochloric acid, add water to dilute to 1000ml, and mix well; medium C, mix 0.05mol / L acetic acid solution-0.05mol / L sodium acetate solution by The volume ratio is 16.4:3.6, and it is ready; for medium D, take 1.7g of potassium dihydrogen phosphate and 1.775g of anhydrous disodium hydrogen phosphate, add an appropriate amount of water to dissolve, and set the volume to 1000mll. Speed: Paddle method 65 rpm
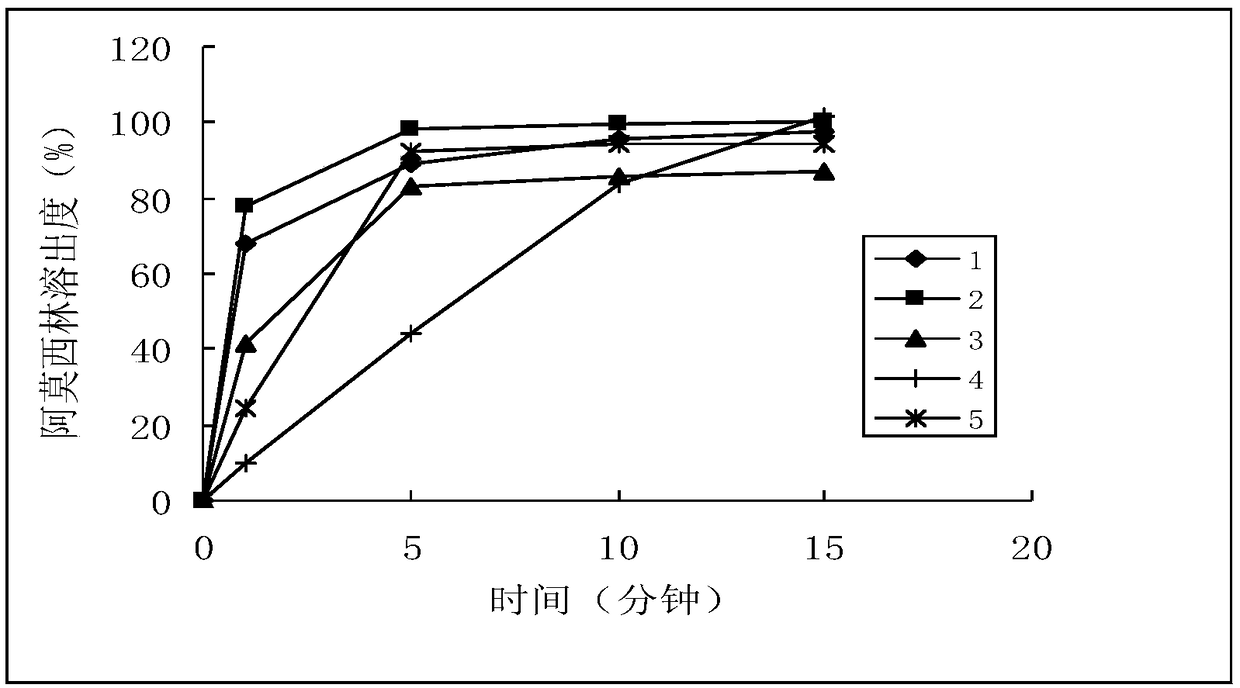
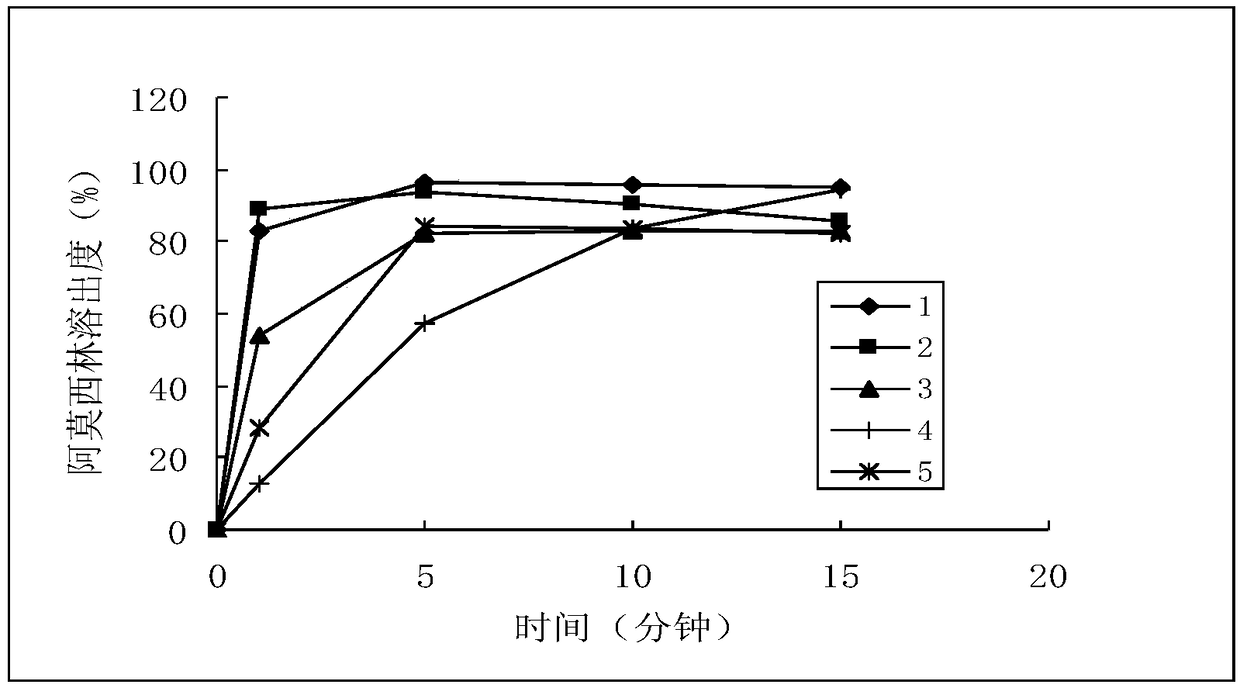
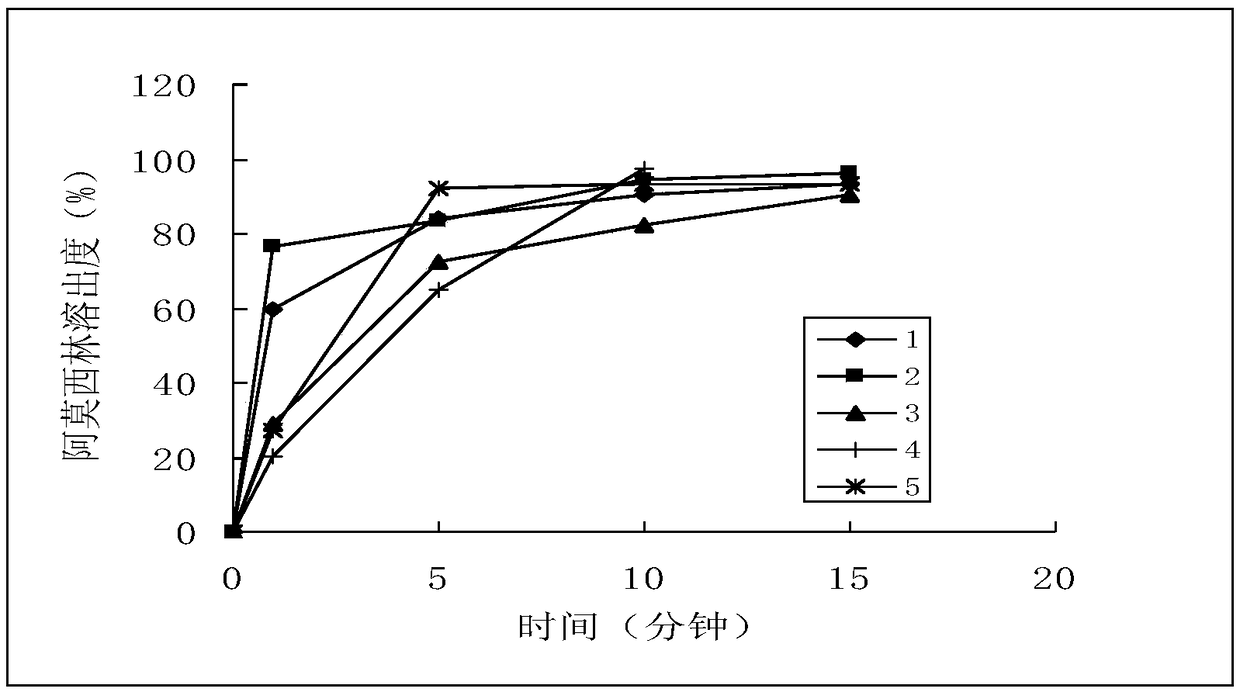
[0040] Sampling time points: 1 minute, 5 minutes, 10 minutes, 15 minutes
[0041] Test solution: take 10ml of dissolution solution at the midpoint between the top o...
Embodiment 2
[0077] 1) System suitability test
[0078] Take 20 μl of the system suitability test reference substance solution and inject it into the liquid chromatograph, and inject it twice continuously. The column efficiency is 13017 based on the amoxicillin peak; the separation degree between the amoxicillin peak and the clavulanic acid peak is 13.34.
[0079] 20 μl of the first reference substance solution was injected continuously for 5 times, and 20 μl of the second reference substance solution was injected continuously for 2 times.
[0080] The RSD% of the amoxicillin peak and the clavulanic acid peak area of the first reference substance solution were both 0.1%; the recovery rate of the second reference substance solution and the first reference substance solution was 99.7%.
[0081] (2) Solution stability
[0082] Use medium A, medium B, medium C and medium D to prepare the test solution according to the law, and place them at 8°C, normal temperature, and 37°C respectively. ...
Embodiment 3
[0092] (1) Selection of dissolution medium
[0093] a) Amoxicillin, dissociation constant (25°C), pKa 1 =2.6 (for carboxyl, adopt titration method to measure); pKa 2 =7.3 (for amino group, adopt titration method to measure); pKa 3 =9.7 (measured by titration method for the alcoholic hydroxyl group of phenol). Solubility in each dissolution medium (37°C), pH 1.2: 26.6mg / ml; pH 4.0: 3.3mg / ml; pH 6.8: 4.3mg / ml; water: 3.1mg / ml.
[0094] Determine to adopt pH1.2 solution, pH4.0 buffer solution, pH6.8 buffer solution and water four kinds of solutions, each 900ml is used as dissolution medium. b) Potassium clavulanate, dissociation constant (25°C): pKa=2.36; solubility in each dissolution medium (37°C), pH4.0: 2.5g / ml; pH 6.8: 2.5g / ml; water: 2.5g / ml. Potassium clavulanate was degraded by 80% when sampling in 1 min in the pH1.2 solution, and it was determined that three solutions of pH4.0 buffer, pH6.8 buffer and water were used, and each 900ml was used as the dissolution mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com