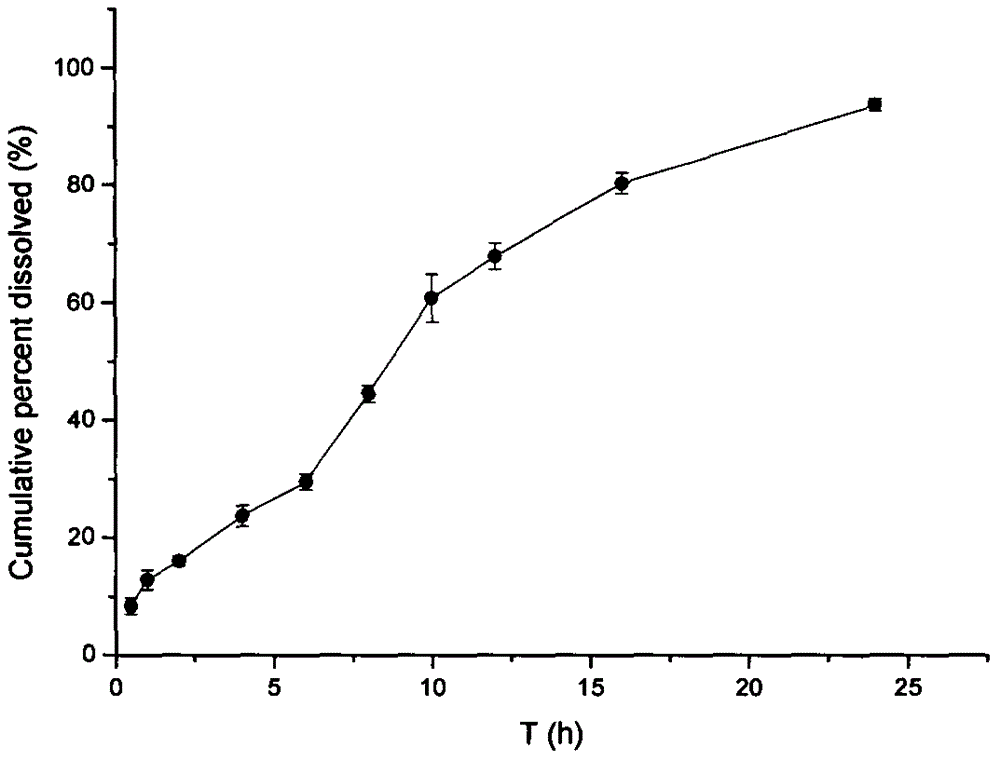
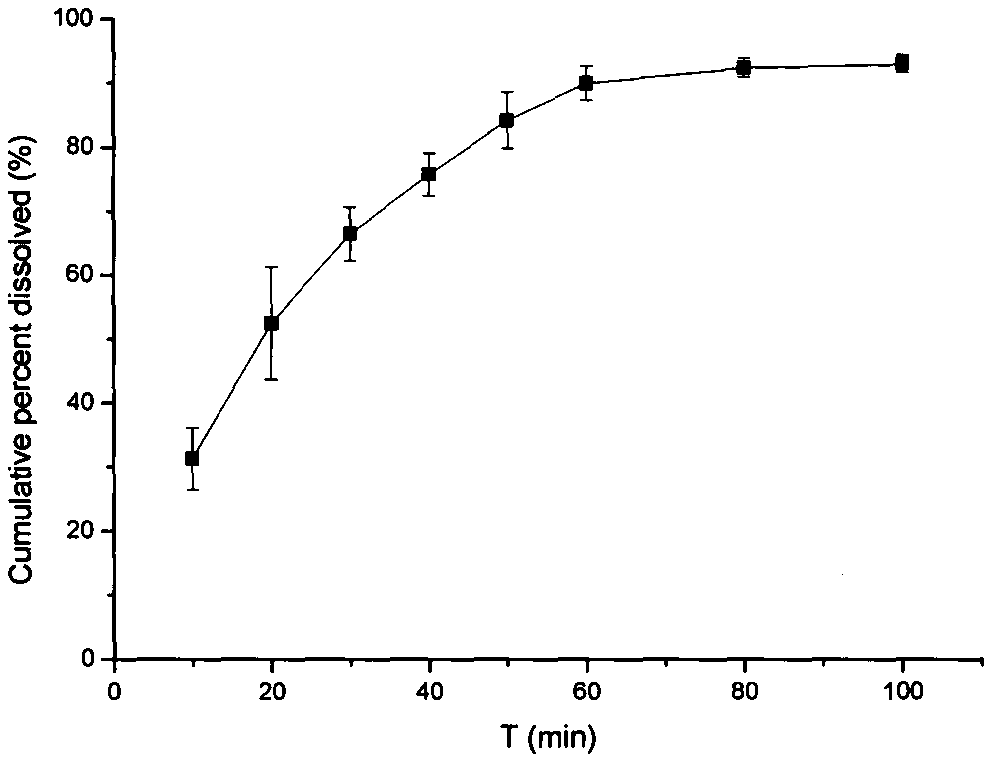
Development for tanshinol-naringenin composite pellet
A technology of danshensu and naringenin, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Preparation of Danshensu sustained-release pellets
[0020] Mix Danshensu and microcrystalline cellulose passed through 80-mesh sieve evenly in a mass ratio of 1:9, and use 5% PVP-K30 as a wetting agent to make a soft material. Put the soft material in the extruder, extrude it into strips at a certain extrusion speed of 10-15rpm, and then transfer the material to the spheronizer for spheronization, set the spheronization speed to 20-25hz and the spheronization time to 5-7min. Finally, the prepared pellets were dried in an oven (40° C. / RH 31%) for 12 hours, sieved through a sieve, and 20-30 mesh pellets were obtained. Surelease ethyl cellulose aqueous dispersion with 15% solid content was prepared as coating liquid. Assemble the fluidized bed, add the danshensu ball core prepared above from above the material barrel of the fluidized bed, and cover with a lid with a mesh. Turn on the blower, adjust the blast temperature to 28-35°C, the blast speed to 20-26Hz, and pr...
Embodiment 2
[0028] (1) Preparation of Danshensu sustained-release pellets
[0029] Mix danshensu and microcrystalline cellulose passed through 80 mesh sieve evenly according to the mass ratio of 1:1, 1:5 and 1:10, make a soft material with 5% PVP-K30 as a wetting agent, and extrude and spheronize Preparation of Danshensu ball core. Prepare 10% ethyl cellulose ethanol solution as the coating solution, and prepare Danshensu sustained-release pellets of various prescriptions according to the method described in Example 1.
[0030] (2) Preparation of naringenin quick-release pellets
[0031] Mix naringenin, microcrystalline cellulose, and sodium carboxymethyl starch through a 80-mesh sieve evenly in a mass ratio of 2:8:2, and use 5% PVP-K30 as a wetting agent to make a soft material. The method described in 1 prepares naringenin quick-release pellets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com