Patents
Literature
56 results about "Deferasirox" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat ongoing high levels of iron in the body caused by multiple blood transfusions. It is also used to treat high levels of iron in people with a certain blood disorder who do not require blood transfusions (non-transfusion-dependent thalassemia).
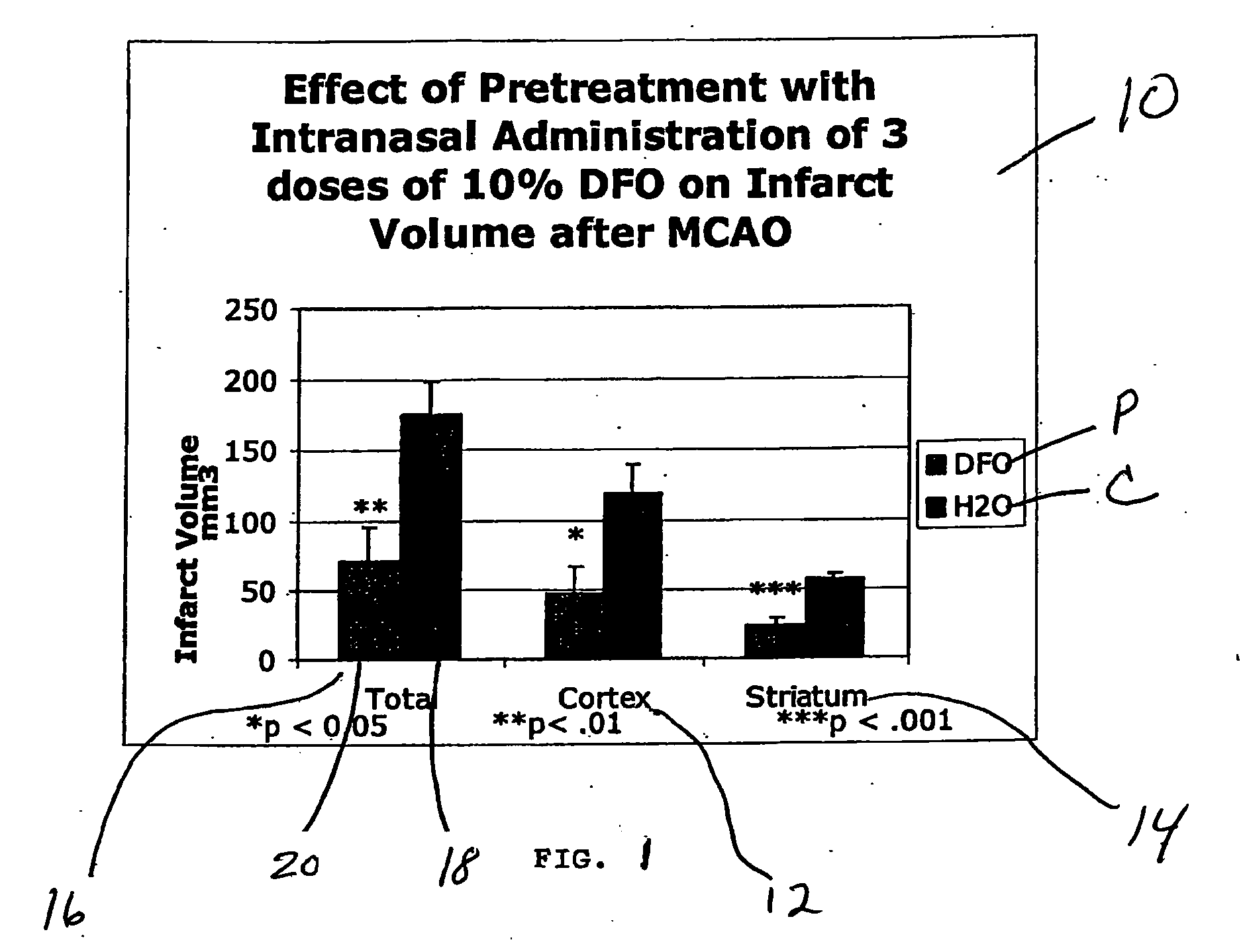
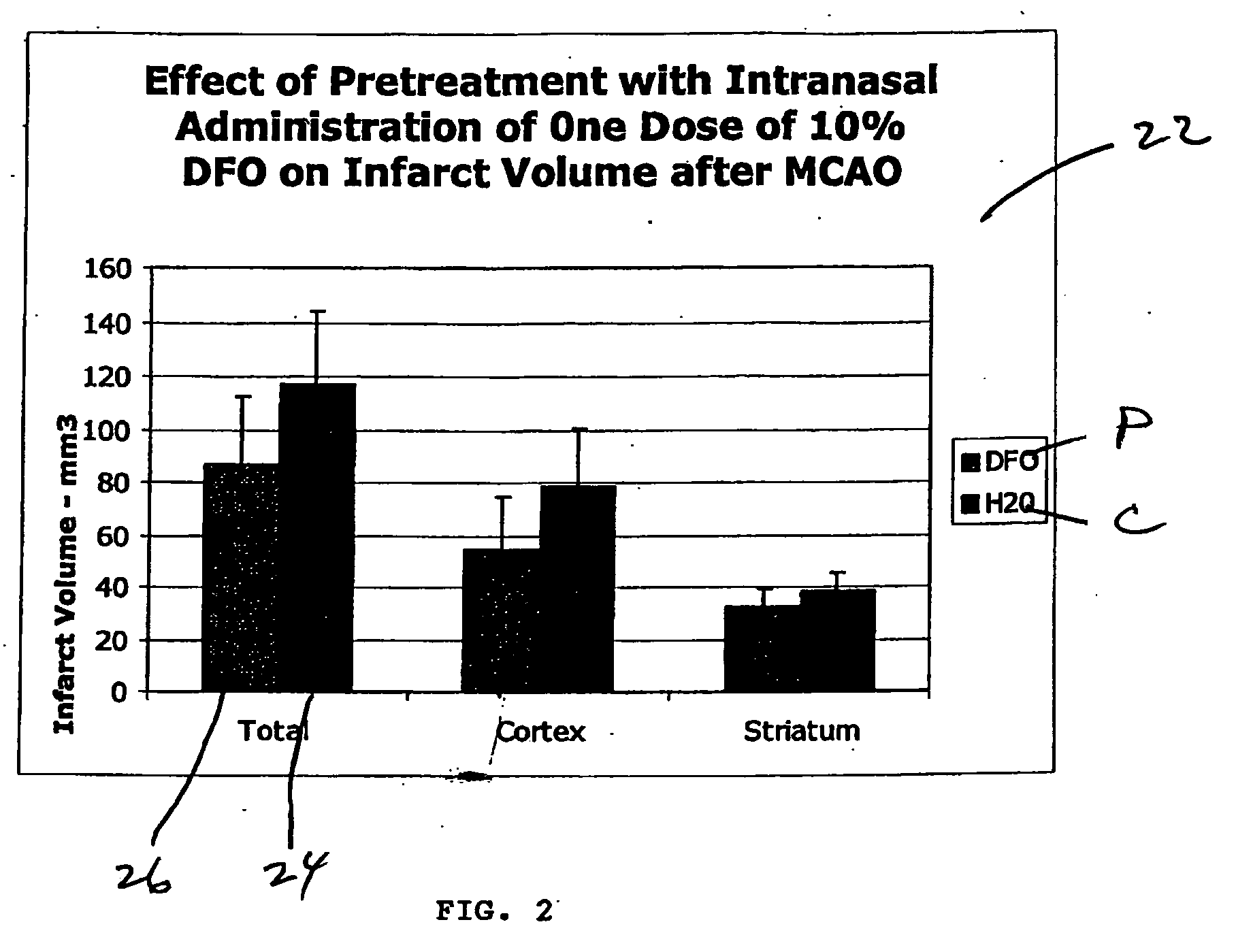
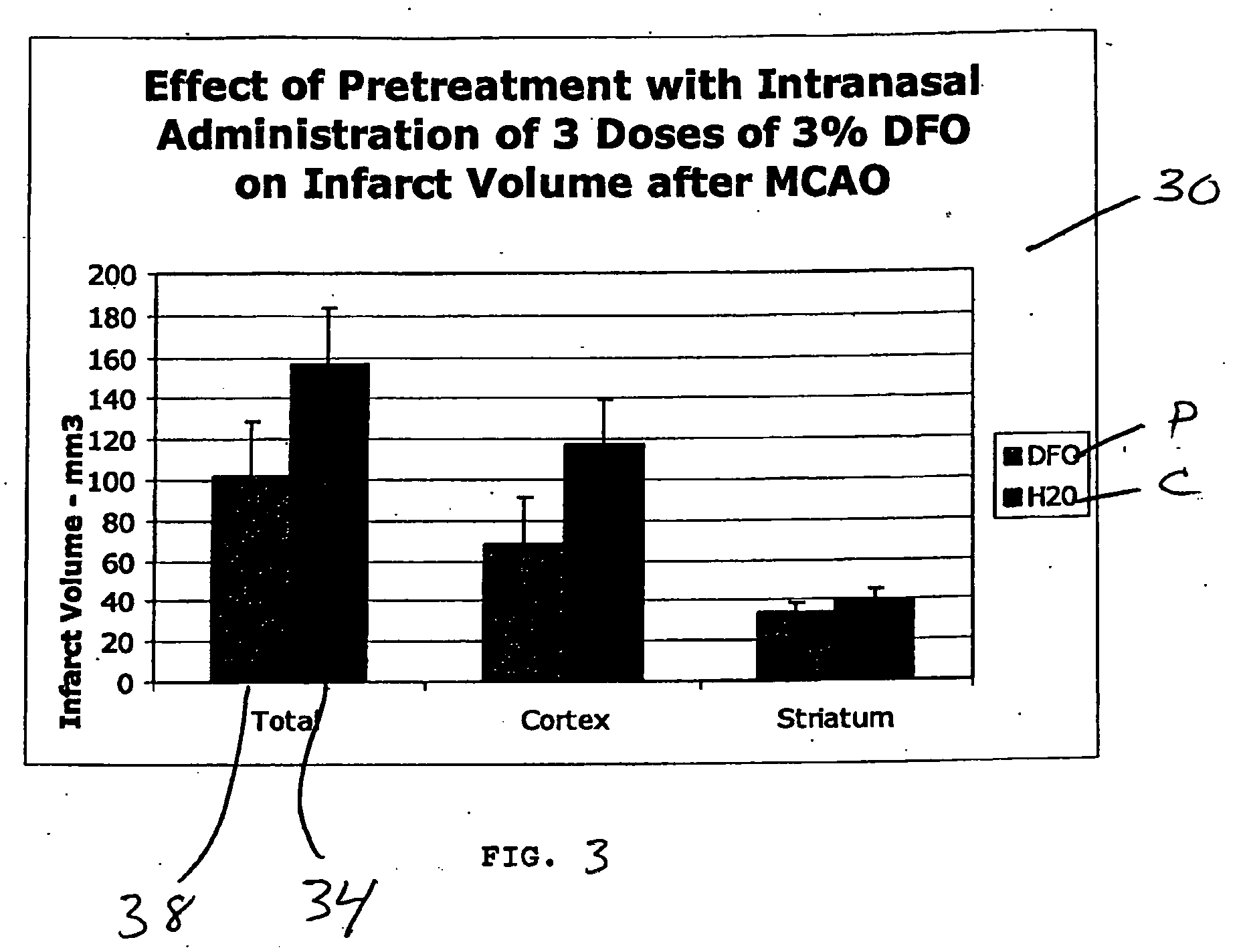
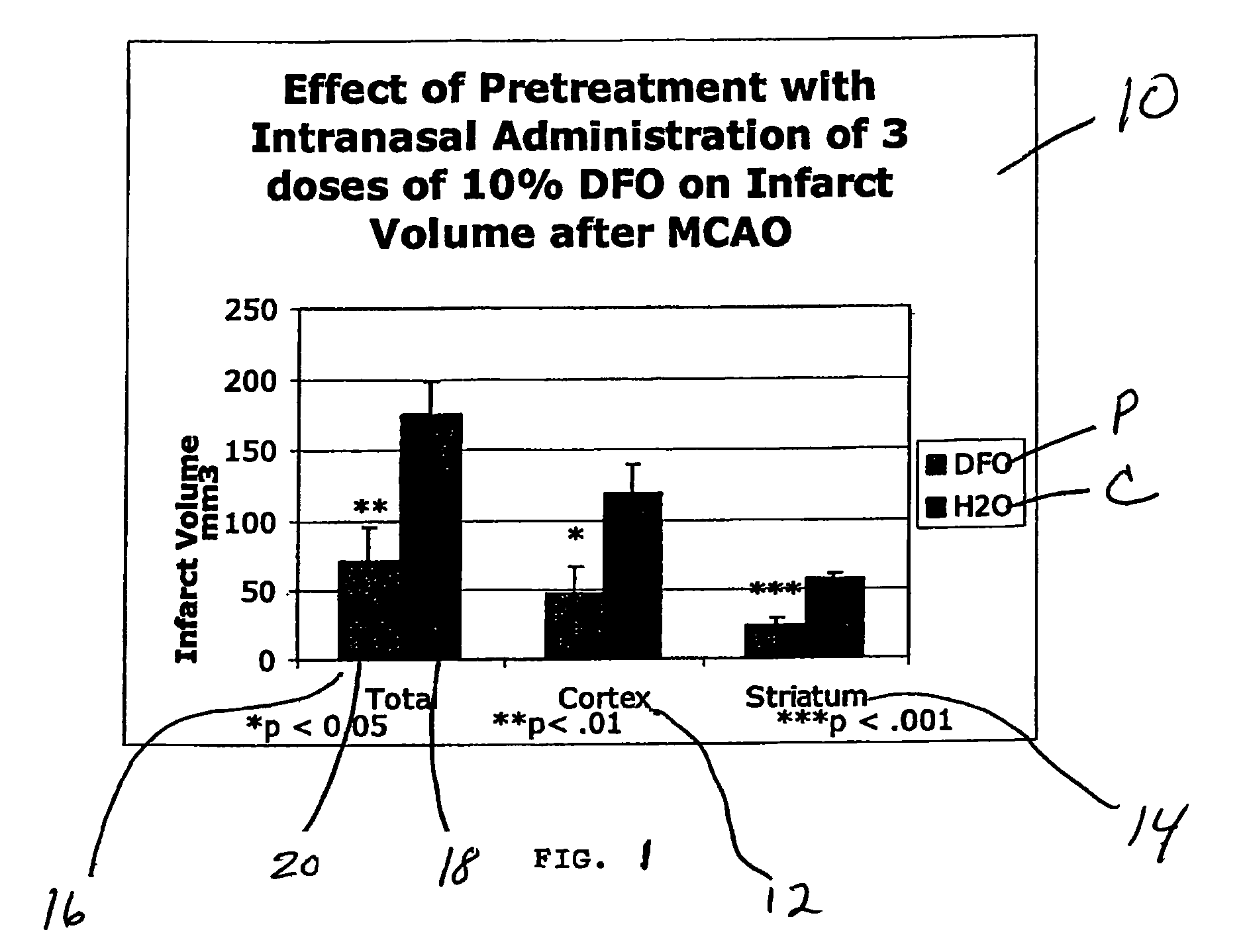
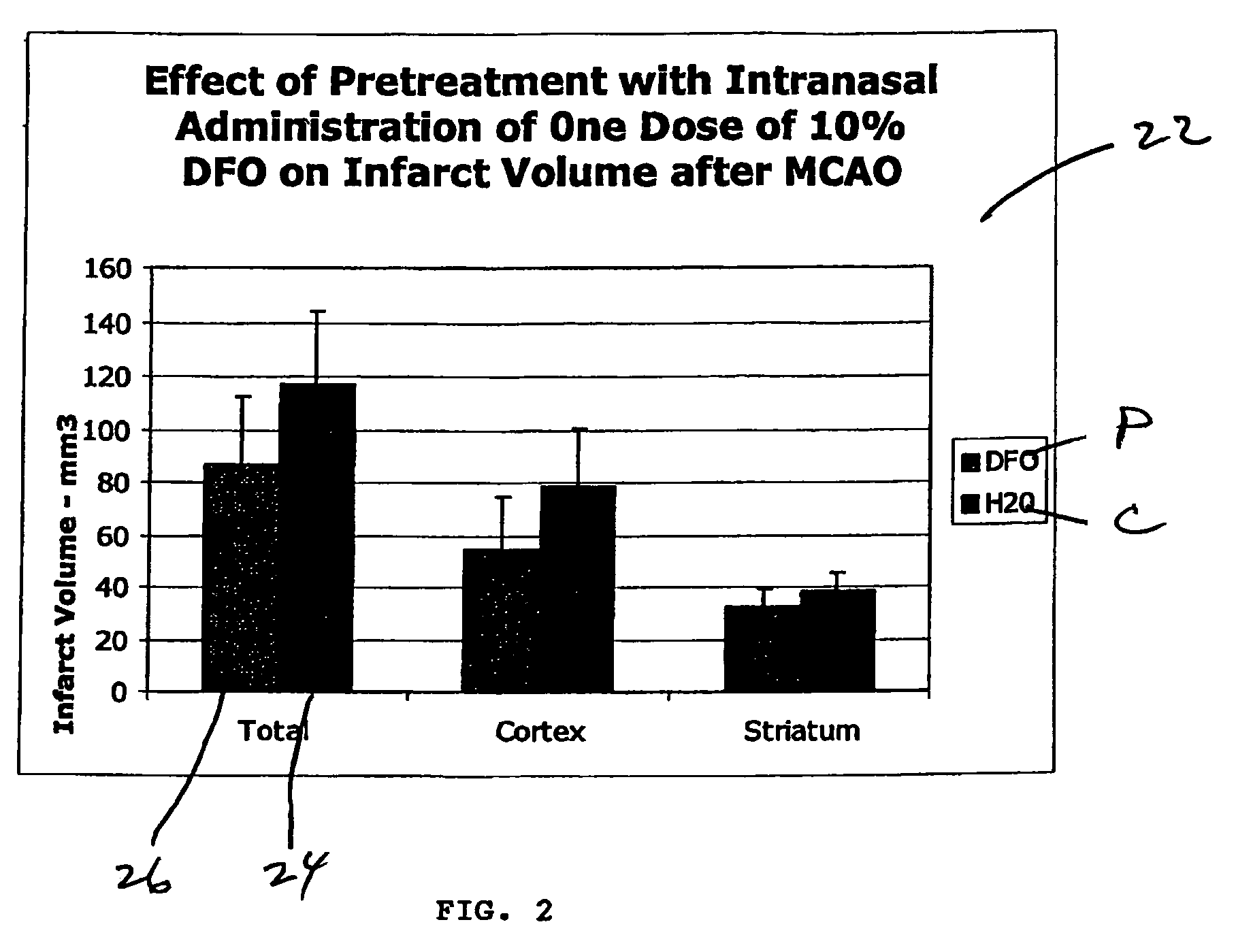
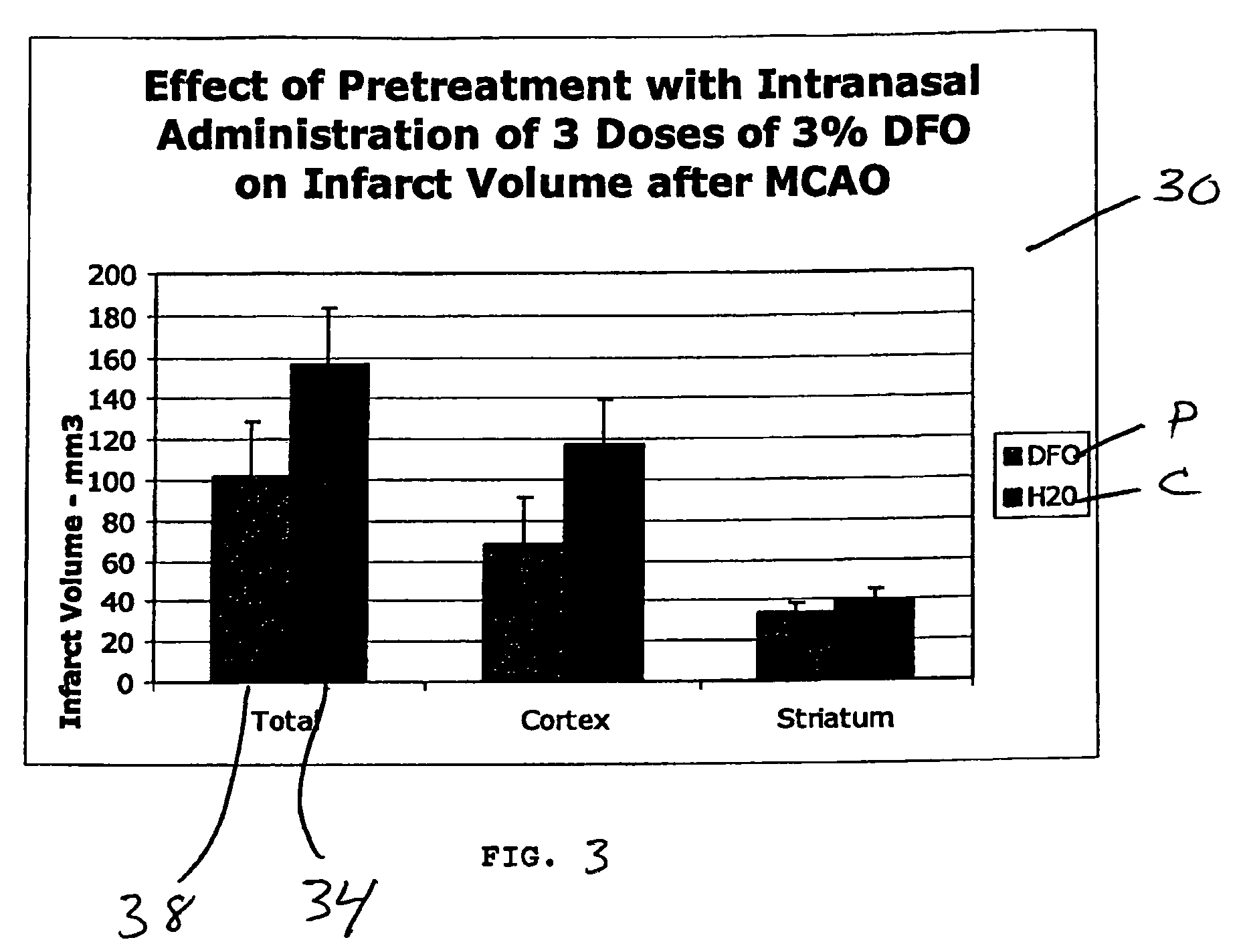
Methods and pharmaceutical compositions for differentially altering gene expression to provide neuroprotection for the animal central nervous system against the effects of ischemia, neurodegeneration, trauma and metal poisoning
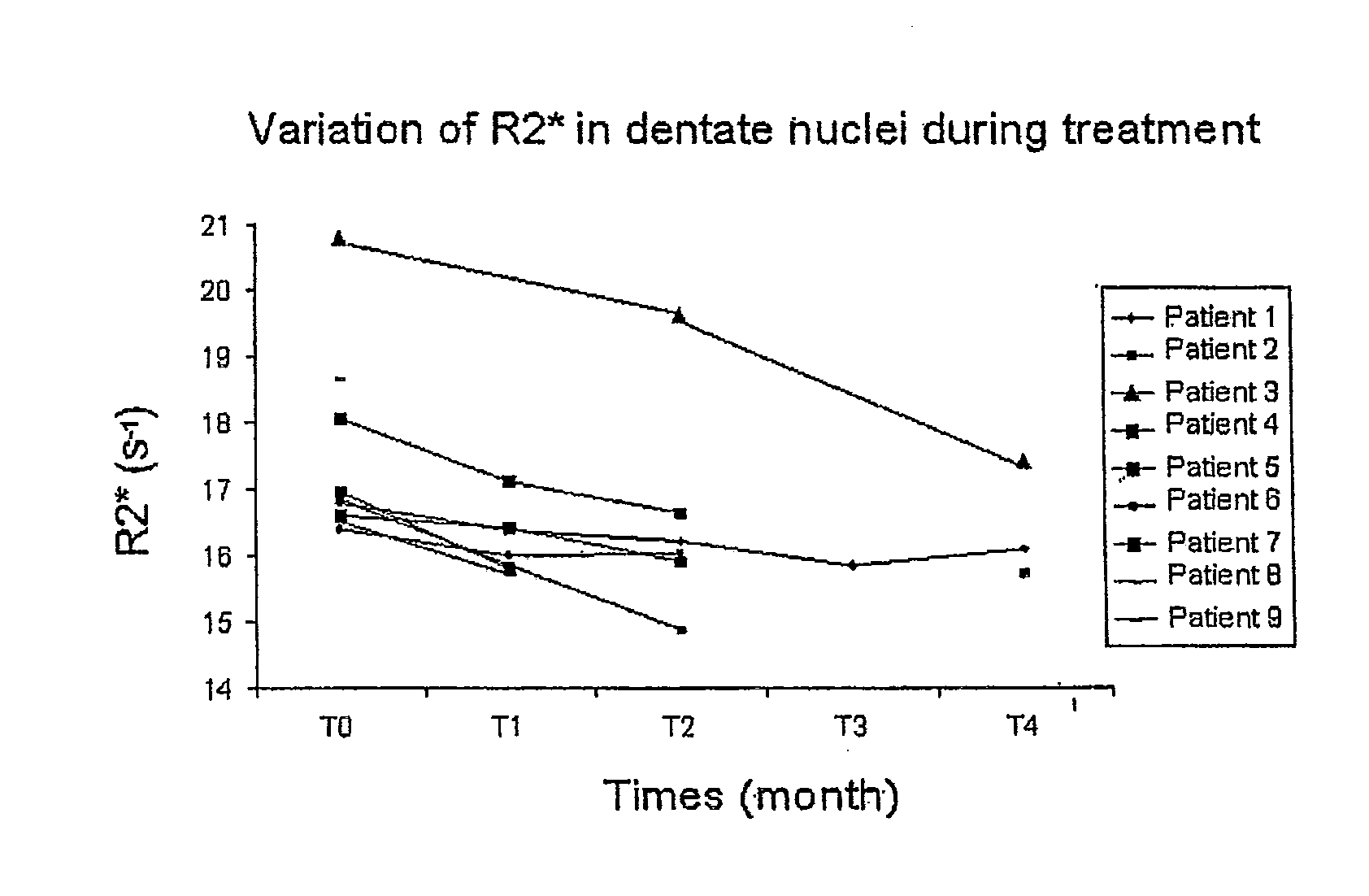
ActiveUS20070092500A1Preventing and minimizing and treating neurologic complicationAvoid side effectsOrganic active ingredientsBiocideAntioxidantNose
Methods and pharmaceutical compositions for preconditioning and / or providing neuroprotection to the animal central nervous system against the effects of neurological disorders involving ischemia, trauma, metal poisoning and neurodegeneration, including the associated cognitive, behavioral and physical impairments. In one embodiment, the method is accomplished by stimulating and / or stabilizing hypoxia-inducible factor-1α (HIF-1α). HIF-1α is known to provide a neuroprotective benefit under ischemic conditions. In another embodiment, the method is accomplished by differentially reducing, inhibiting or preventing the increased expression of selected genes caused by neurological disorders. Patients at risk for certain diseases or disorders that are associated with risk for cerebral ischemia may benefit, e.g., those at risk for Alzheimer's disease, Parkinson's disease, Wilson's disease, Huntington's disease, thalassemia or stroke, or those patients having head or spinal cord injury. Patients undergoing certain medical procedures that may result in ischemia may also benefit. Initially, the possibility of ischemia or neurodegeneration is recognized. Intranasal therapeutic agents are administered to the upper third of the nasal cavity to bypass the blood-brain barrier and access the central nervous system directly to avoid unwanted and potentially lethal side effects. Therapeutic agents include those substances that interact with iron and / or copper such as iron chelators, copper chelators, and antioxidants. Particular examples of such therapeutic agents are the iron chelators deferoxamine (DFO) and deferasirox. Intranasal administration of DFO is known to stimulate and / or stabilize HIF-1α and provides an efficient and safe method for pre-conditioning the brain to protect against cerebral ischemia.
Owner:HEALTHPARTNERS RESEACH FOUND
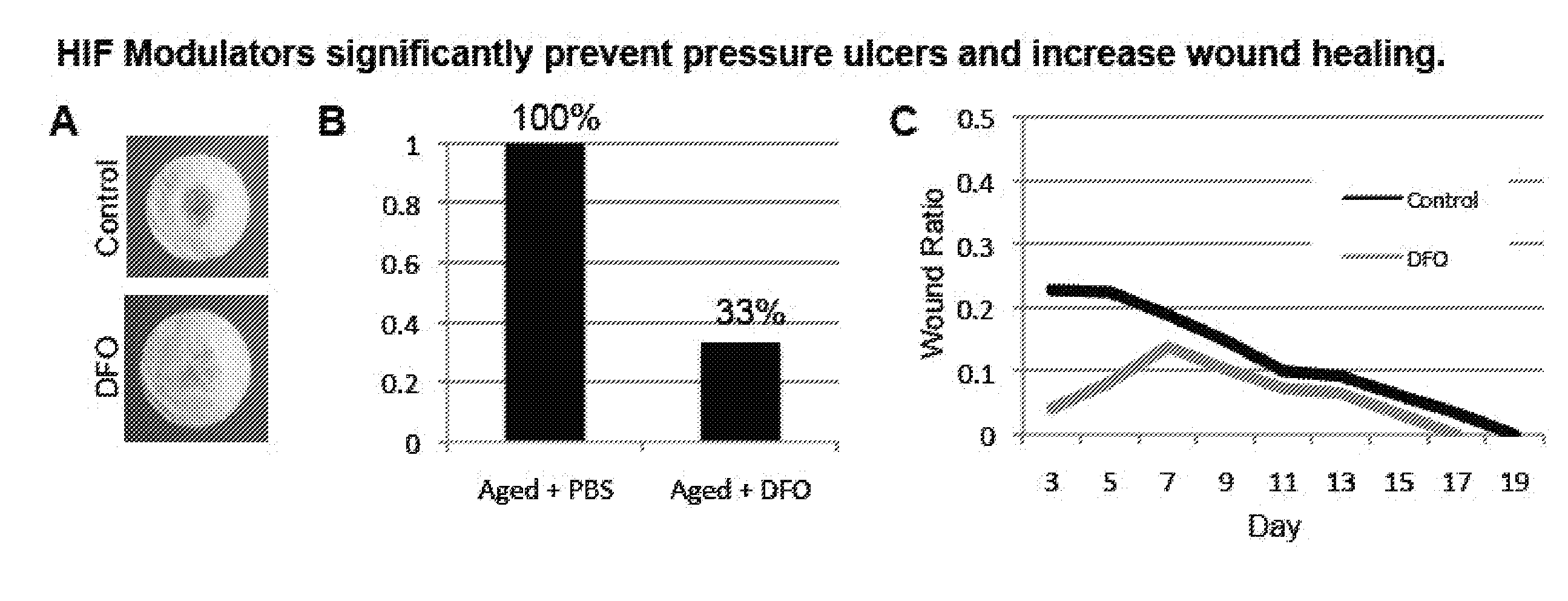
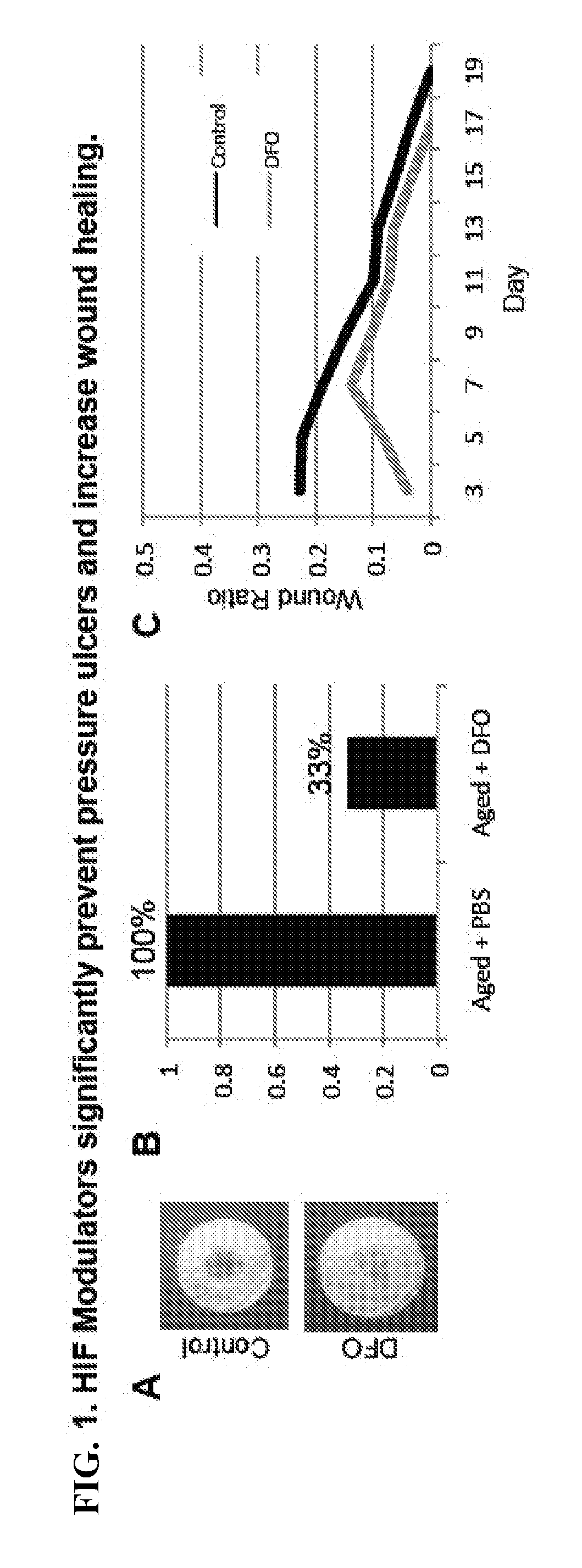
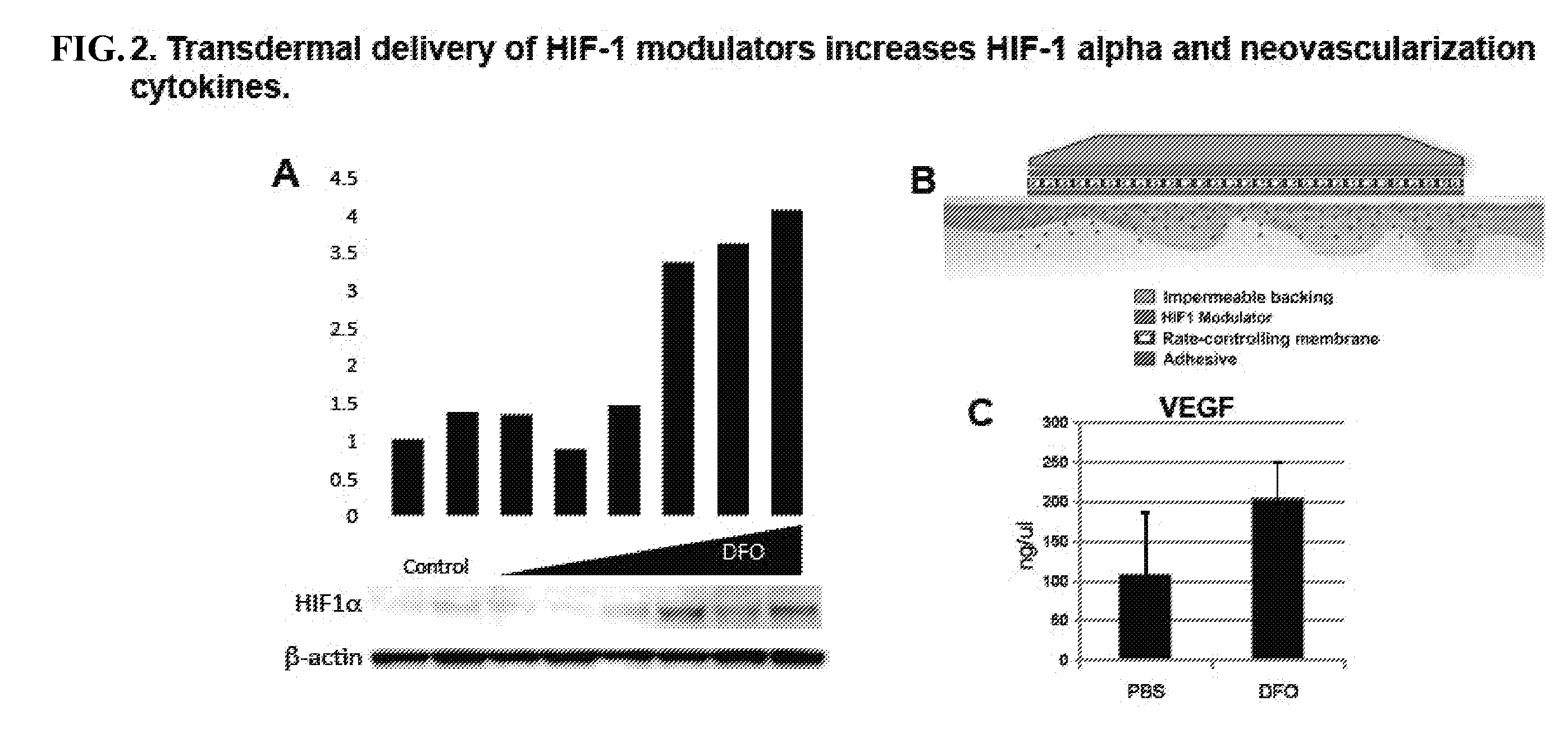
Topical and Transdermal Delivery of HIF-1 Modulators to Prevent and Treat Chronic Wounds
InactiveUS20100092546A1High activityPromote wound healingBiocidePeptide/protein ingredientsChronic woundDeferasirox
Compositions and methods are provided for the treatment of chronic wounds, including, without limitation, pressure ulcers and diabetic ulcers, by transdermal delivery of an agent that increases activity of HIF-1α in the wound. Agents that increase HIF-1α activity include, without limitation, agents that stabilize HIF-1α, e.g. deferoxamine, deferiprone, deferasirox, etc.; agents that upregulate expression of HIF-1α, e.g. dimethyloxalylglycine, etc., HIF-1α polypeptide or coding sequences; and combinations thereof. Such agents may be referred to herein as HIF-1α potentiating agents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Use of deferiprone and methods to treat and/or prevent friedreich ataxia resulting from intracellular mishandling of iron
A therapeutically effective amount of deferiprone or deferasirox or physiologically acceptable salts thereof for the prevention, stabilization, treatment, or reversal of iron-induced FRDA disease in patients resulting from mitochondrial iron-induced damage to preferentially reduce the iron stores in the mitochondria. Also for the treatment of other conditions affecting the brain where a key element in the generation of the resultant pathology is the intracellular mishandling of iron.
Owner:MUNNICH ARNOLD +2
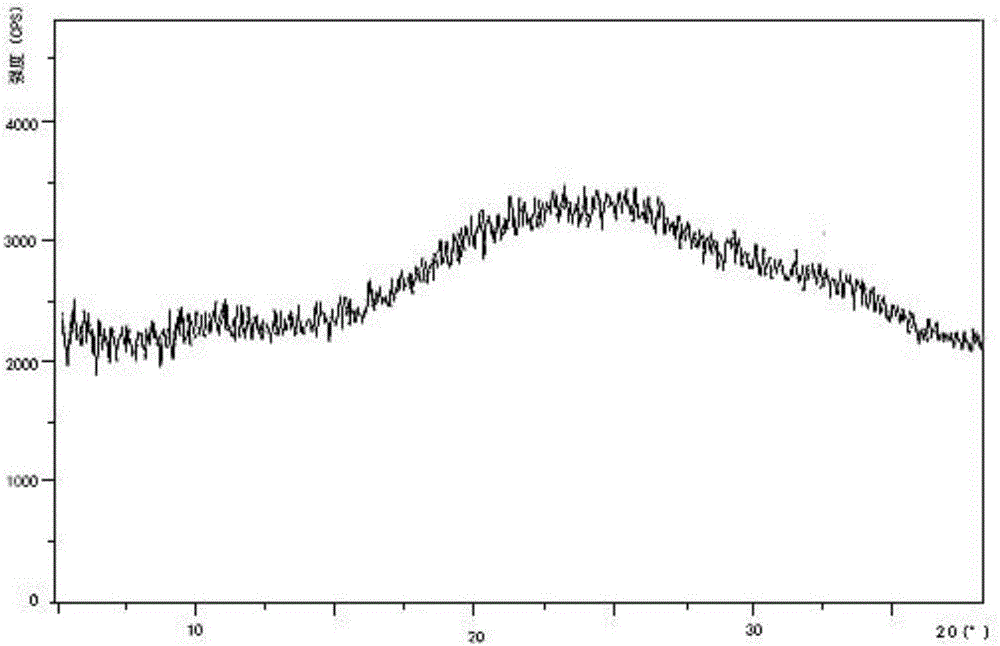
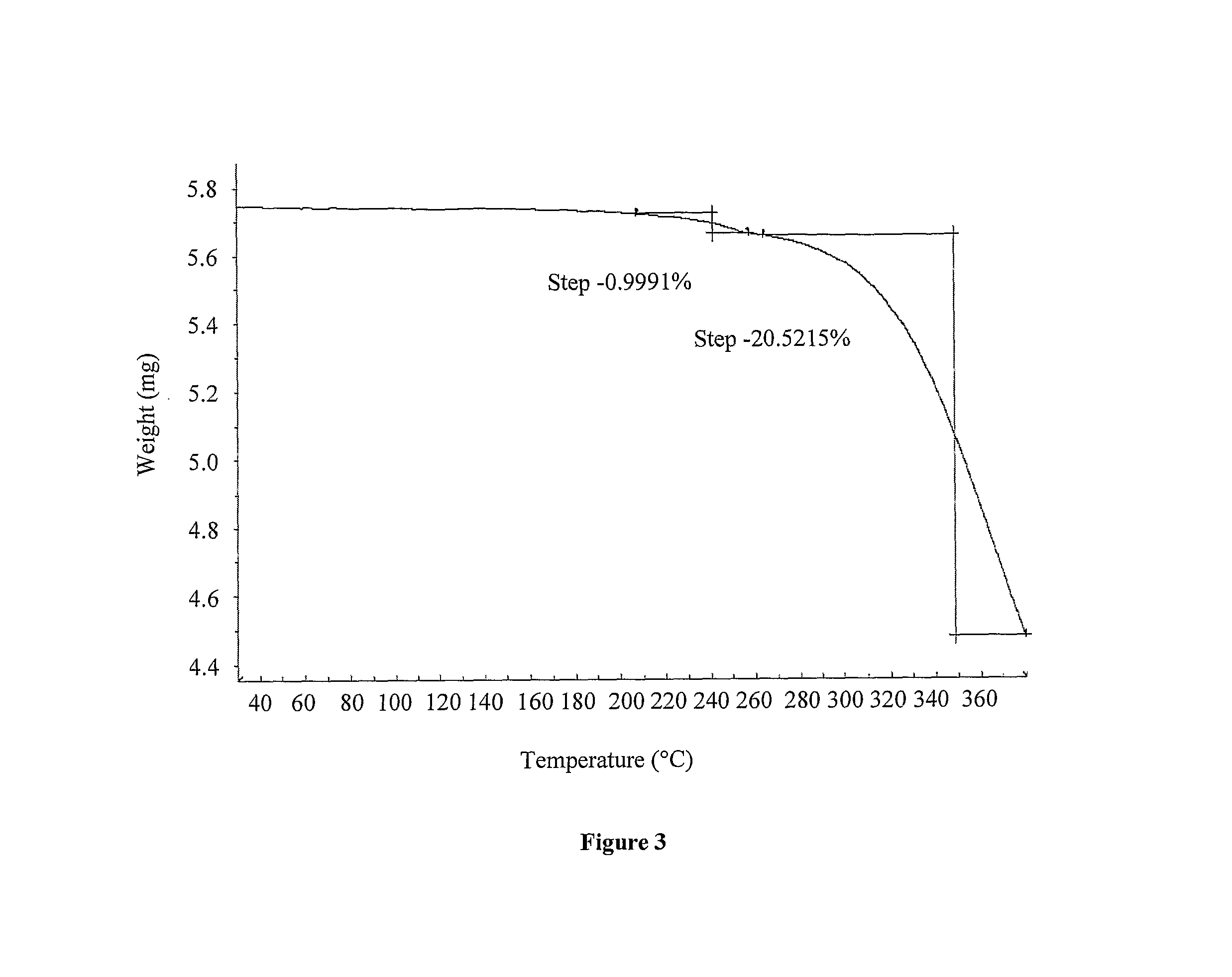
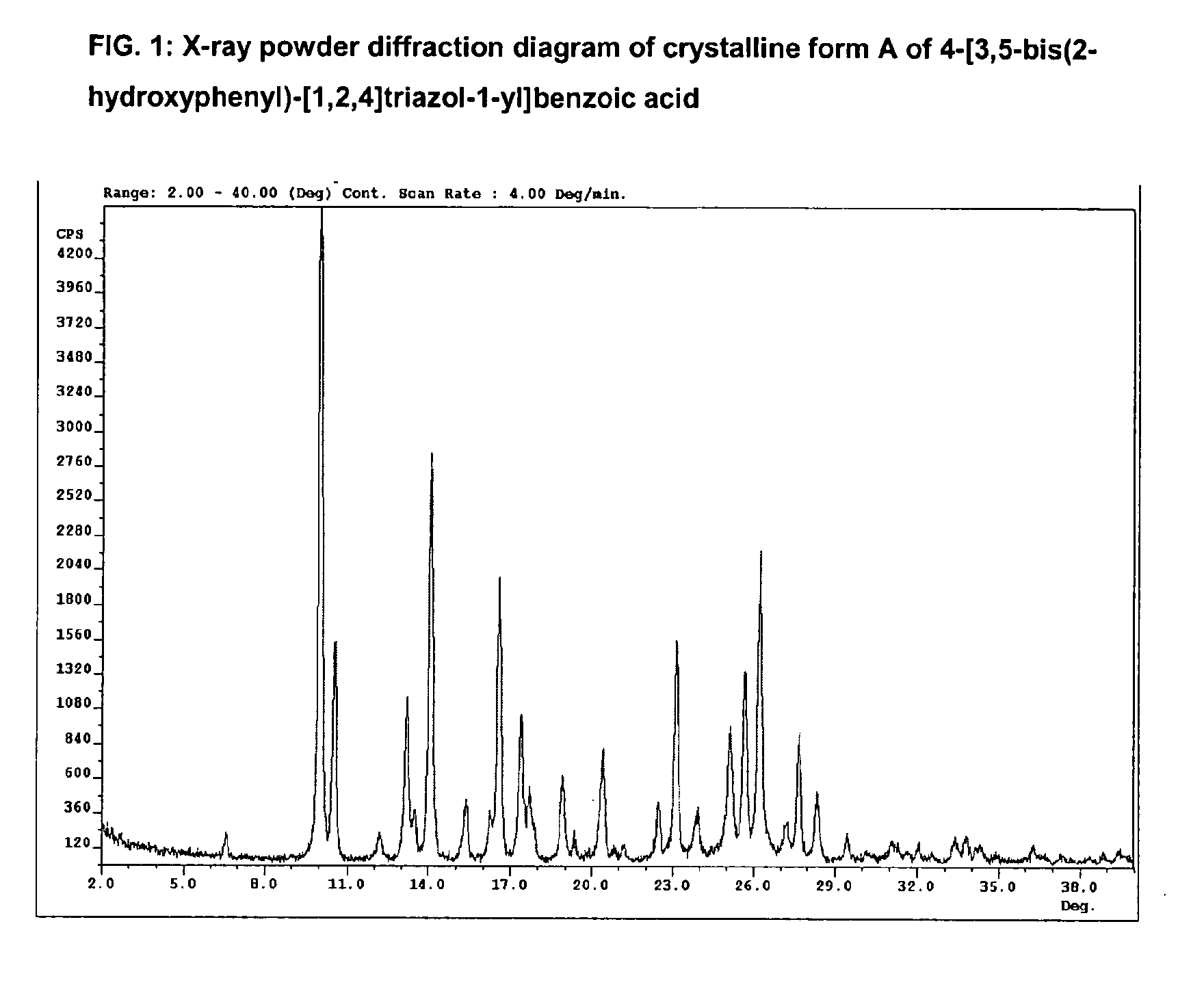
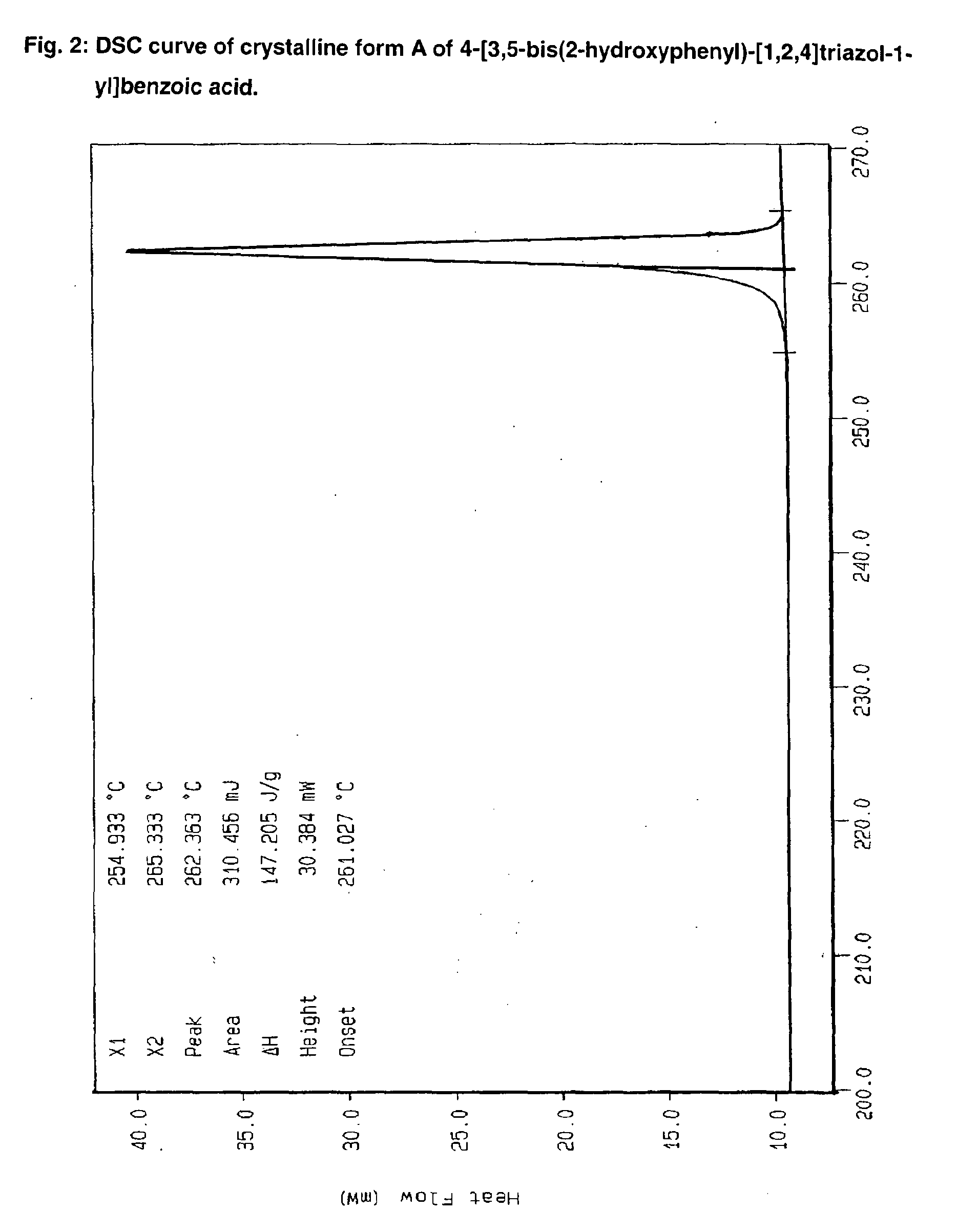
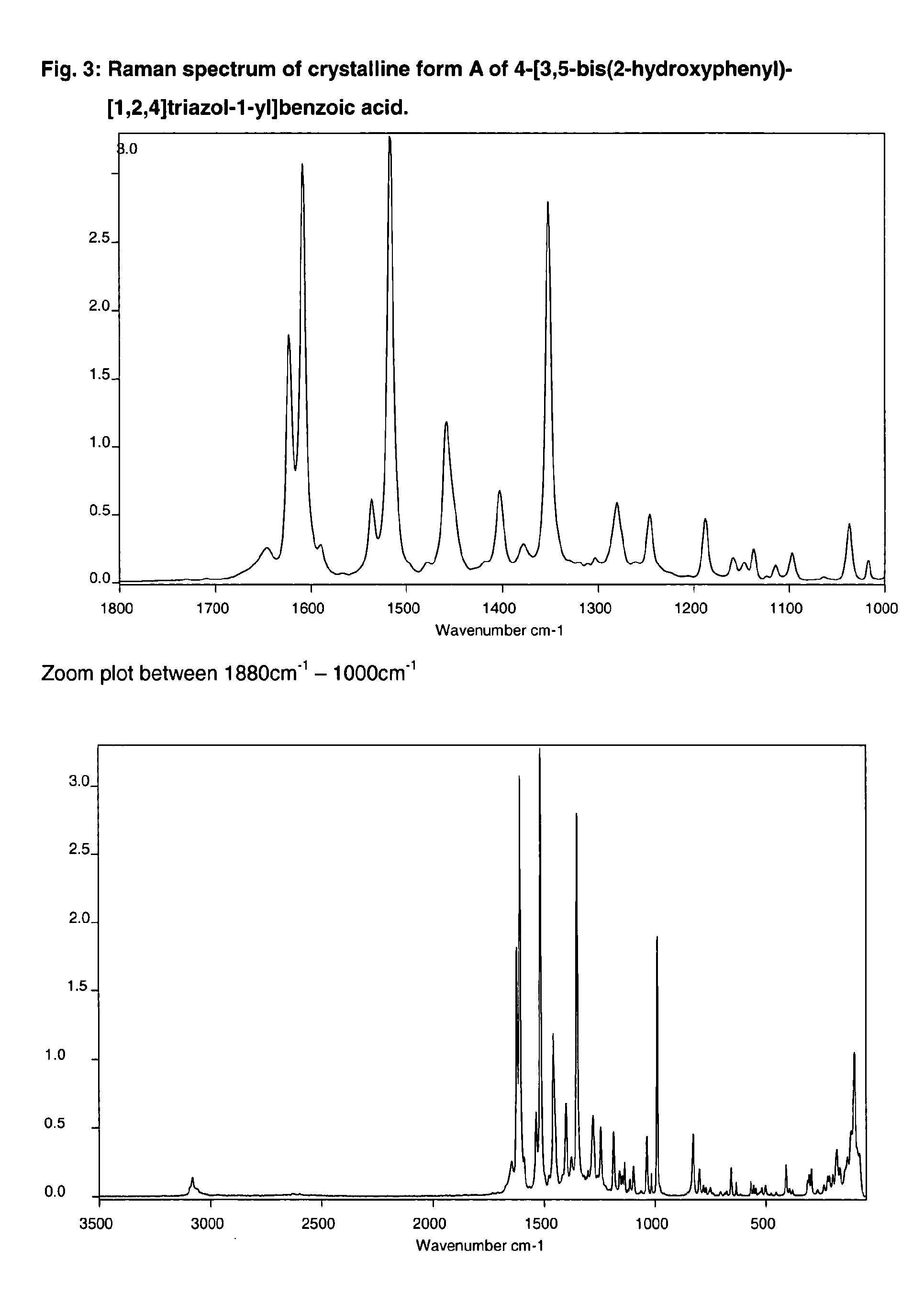
Processes for the preparation of deferasirox, and deferasirox polymorphs
The present invention relates to processes for the preparation of deferasirox, an oral iron chelator developed to treat iron overload due to e.g. multiple blood transfusions. The present invention further provides novel deferasirox pseudopolymorphs and a novel amorphous form of deferasirox, processes for their preparation, as well as pharmaceutical compositions comprising same, and use thereof in treating iron overload.
Owner:MAPI PHARMA
Compositions and methods for the treatment of mucormycosis and other fungal diseases
The invention provides a composition including at least one iron chelating compound and at least one antifungal agent. The composition can include the iron chelating compounds deferiprone or deferasirox. An antifungal agent included in the composition can include a polyene antifungal agent, an azole antifungal agent or an echinocandin antifungal agent. The invention also provides a method of treating or preventing a fungal condition. The method includes administering to an individual having, or susceptible to having, a fungal condition a therapeutically effective amount of at least one iron chelating compound for a sufficient time to reduce the severity of a fungal condition, wherein the iron chelating compound comprises a non-siderophore or non-xenosiderophore relative to the fungal condition. A method of treating or preventing a fungal condition provided by the invention also can include administering to an individual having, or susceptible of having, a fungal condition a therapeutically effective amount of at least one iron chelating compound and at least one antifungal agent. Provided further by the invention is a method including prophylactic administration of the at least one iron chelating compound or at least one iron chelating compound and at least one antifungal agent prior to onset of the fungal condition.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
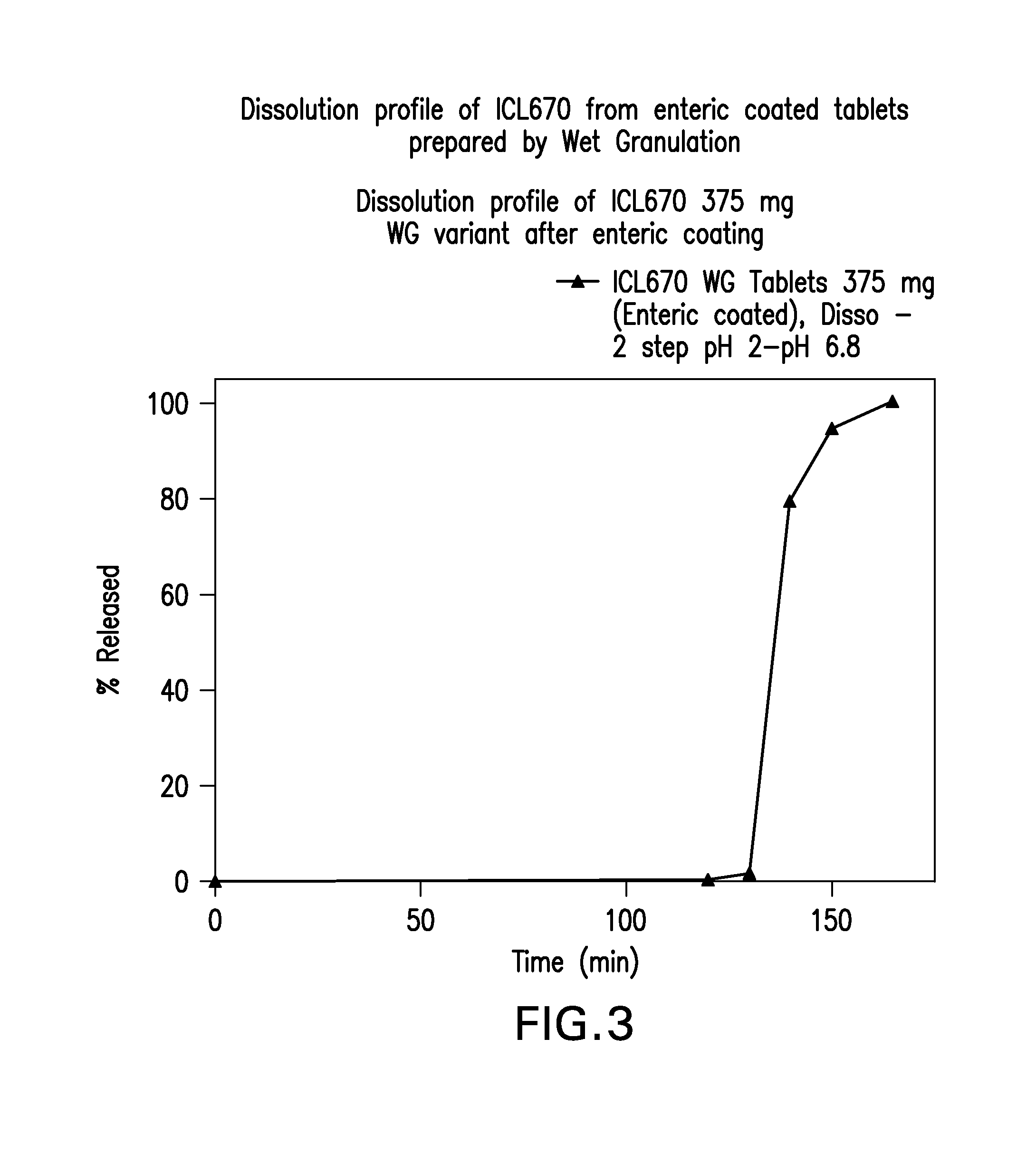
Oral formulations of deferasirox
Orally administerable deferasirox formulations are disclosed having reduced release under gastric conditions and fast release at near neutral pH or at neutral pH.
Owner:NOVARTIS AG
Method of treating Alzheimer's disease comprising administering deferoxamine (DFO) to the upper one-third of the nasal cavity
ActiveUS7776312B2Avoid side effectsStimulate and stabilize HIF-1αBiocideOrganic active ingredientsNervous systemNose
Owner:HEALTHPARTNERS RESEACH FOUND
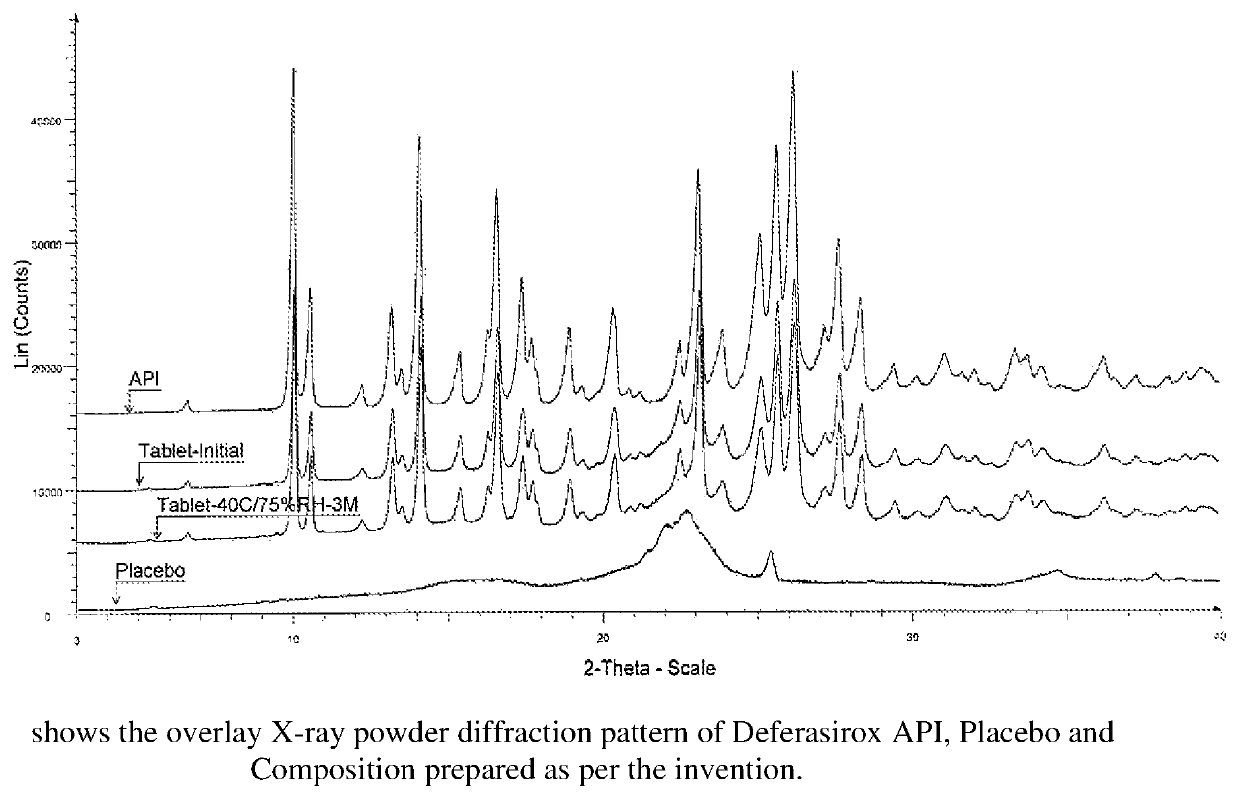
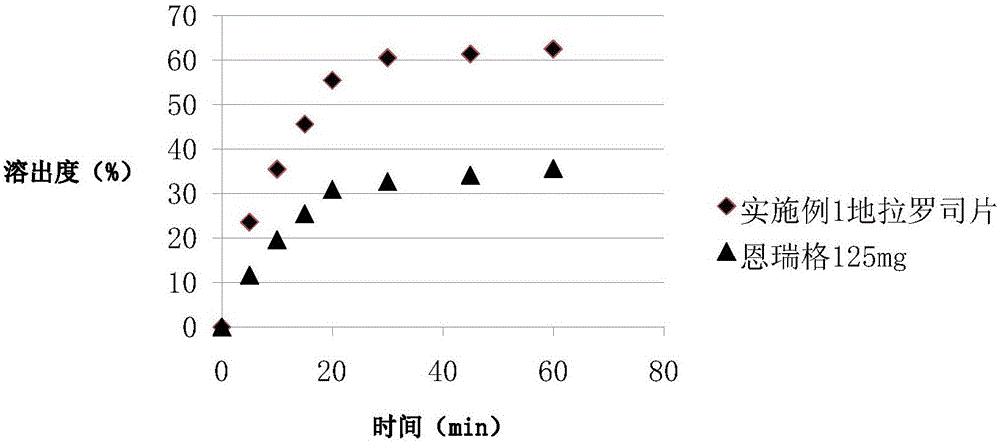
Pharmaceutical Composition Comprising Deferasirox
InactiveUS20140147503A1Improve solubilityIncrease surface areaPowder deliveryBiocideSolubilityDeferasirox
The present invention relates to a pharmaceutical composition comprising deferasirox, a process for preparing such pharmaceutical composition, and its use in the treatment of chronic iron overload. The pharmaceutical composition comprises nanosized deferasirox having improved surface area and solubility. It also relates to a method for treatment of chronic iron overload which comprises administering a pharmaceutical composition comprising nanosized deferasirox.
Owner:CIPLA LTD
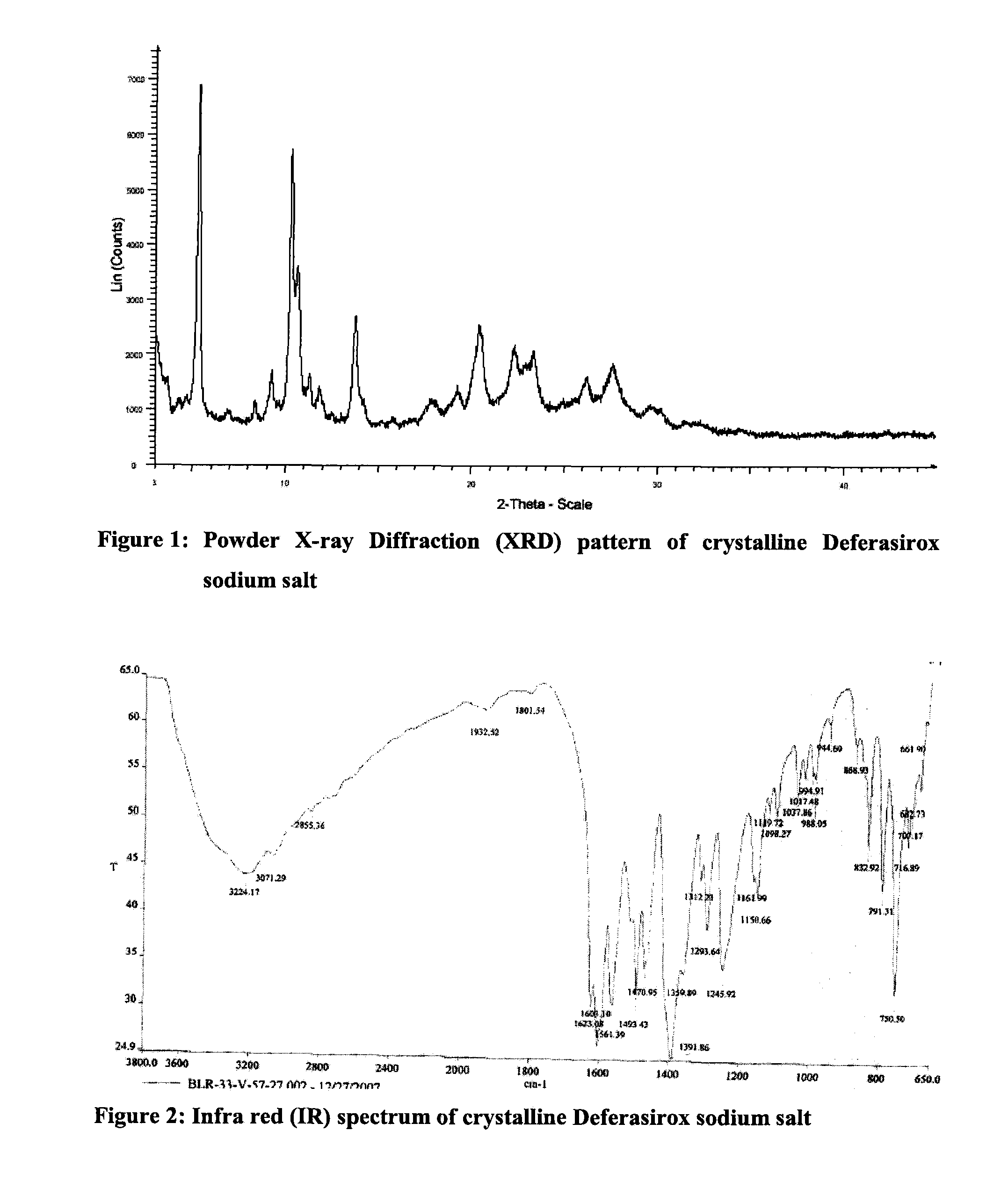
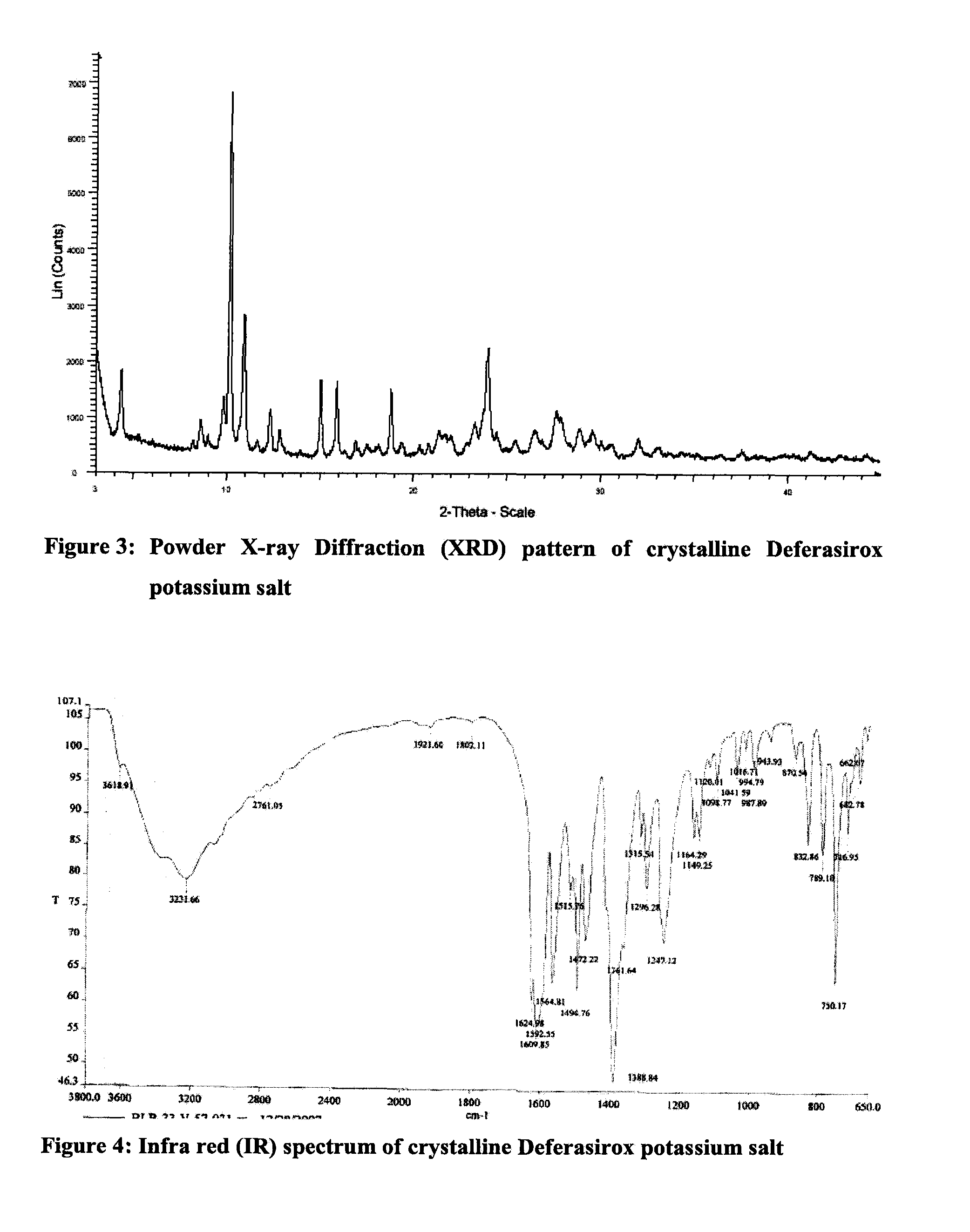
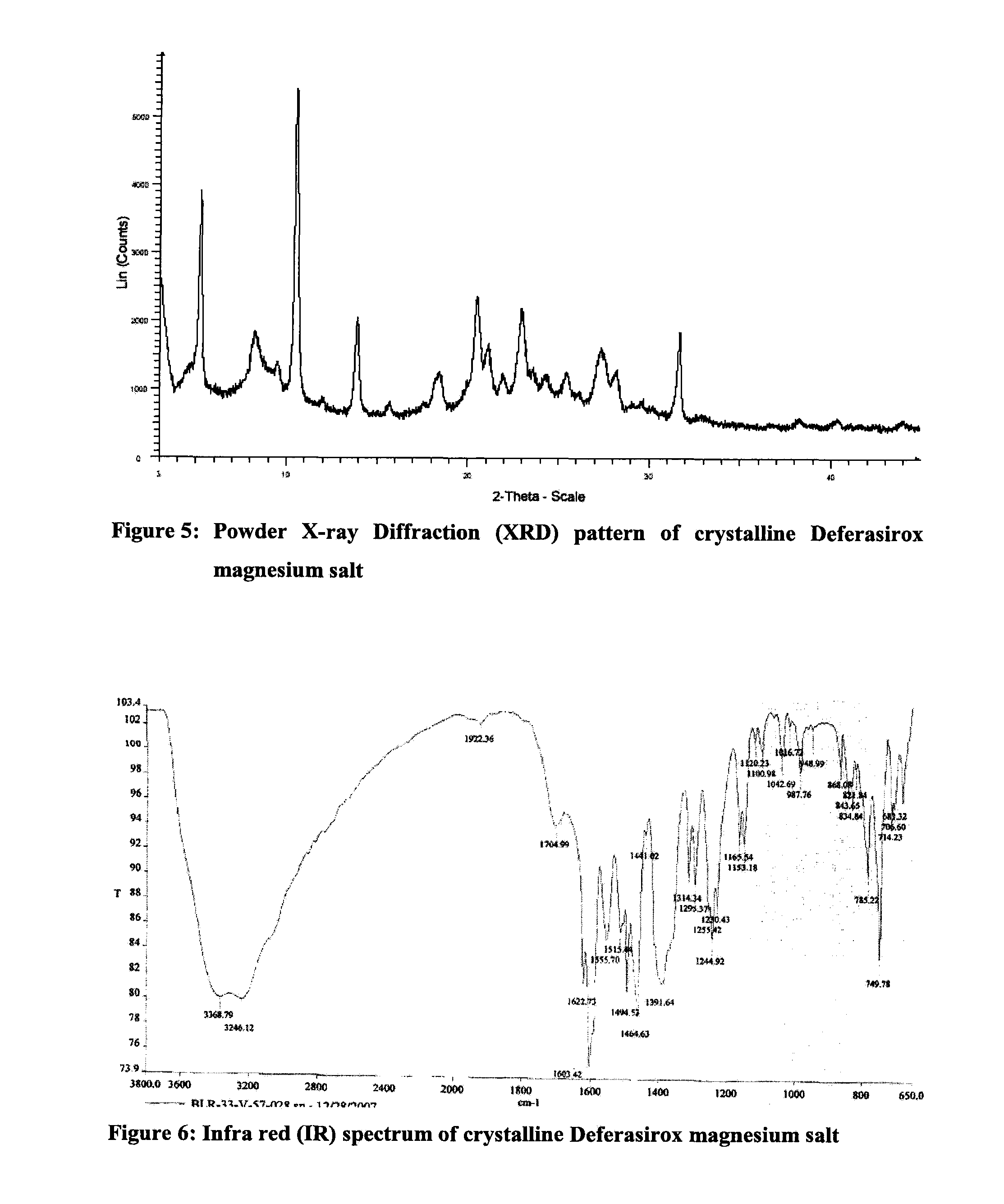
Solid state forms of deferasirox salts and process for the preparation thereof
InactiveUS20110097413A1Improve liquidityHigh purityBiocidePowder deliveryDeferasiroxMedicinal chemistry
Provided herein are novel solid state forms of deferasirox salts, process for the preparation, pharmaceutical compositions, and method of treating thereof. The solid state forms of deferasirox salts are useful for preparing deferasirox (I) in high purity.
Owner:ACTAVIS GRP PTC EHF
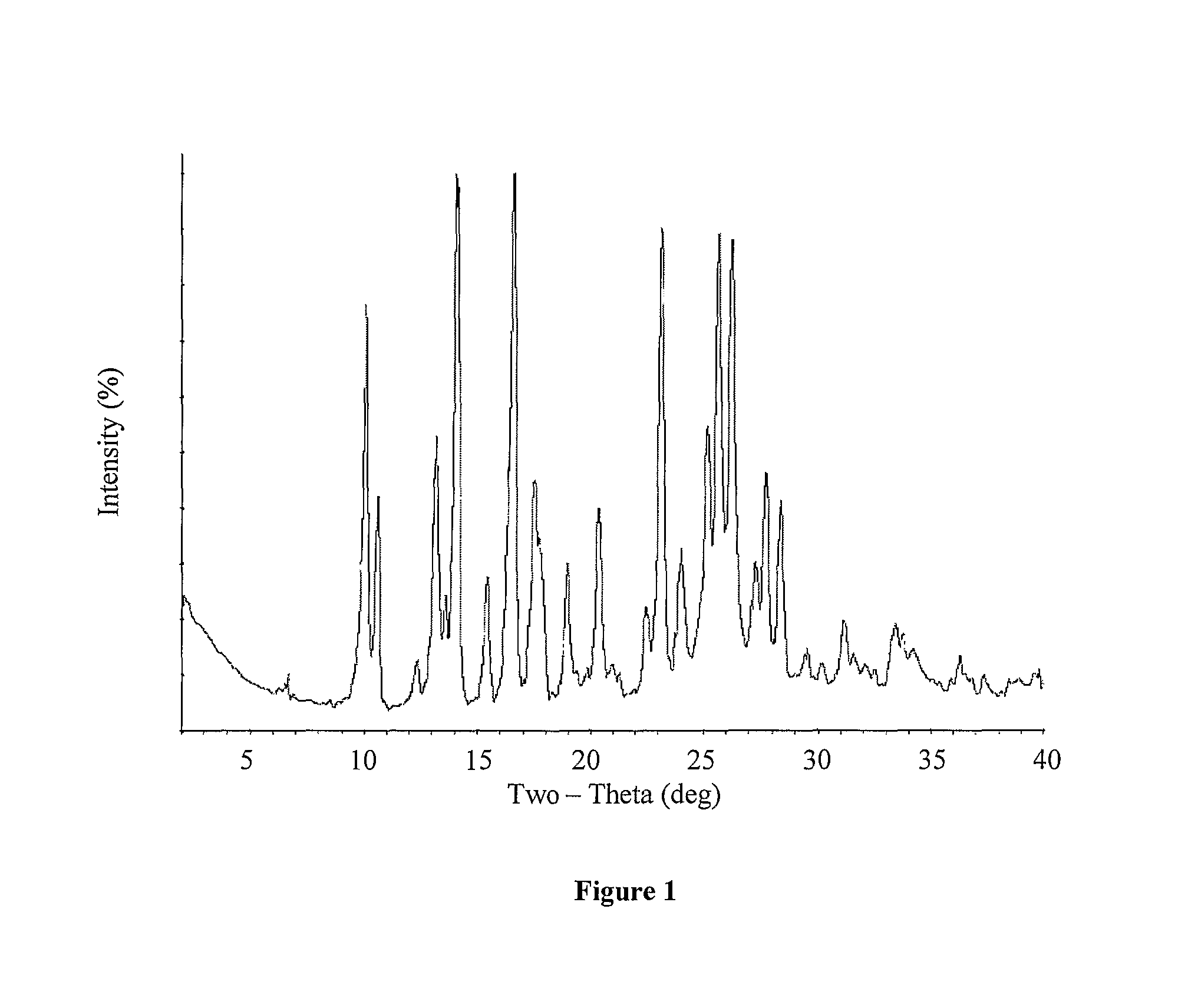
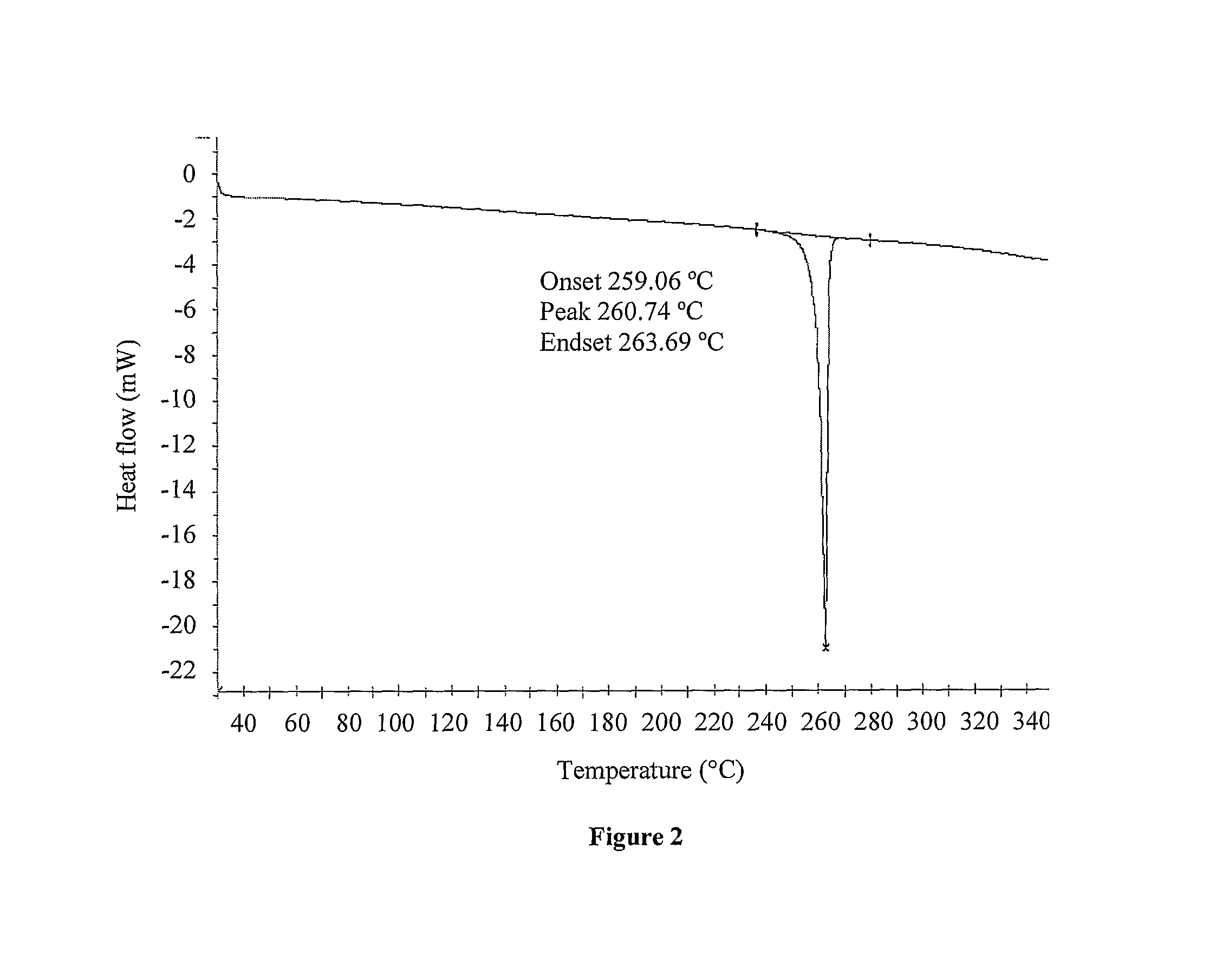
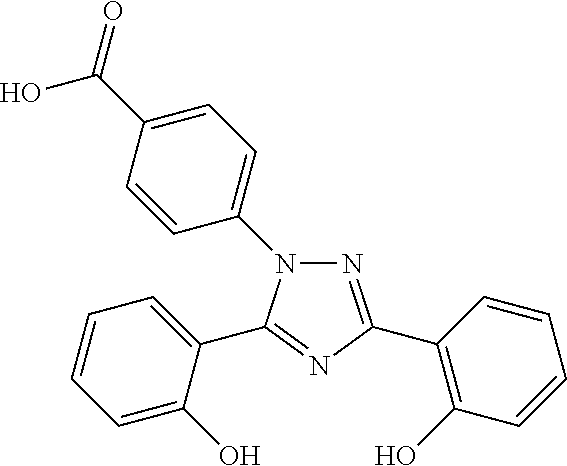
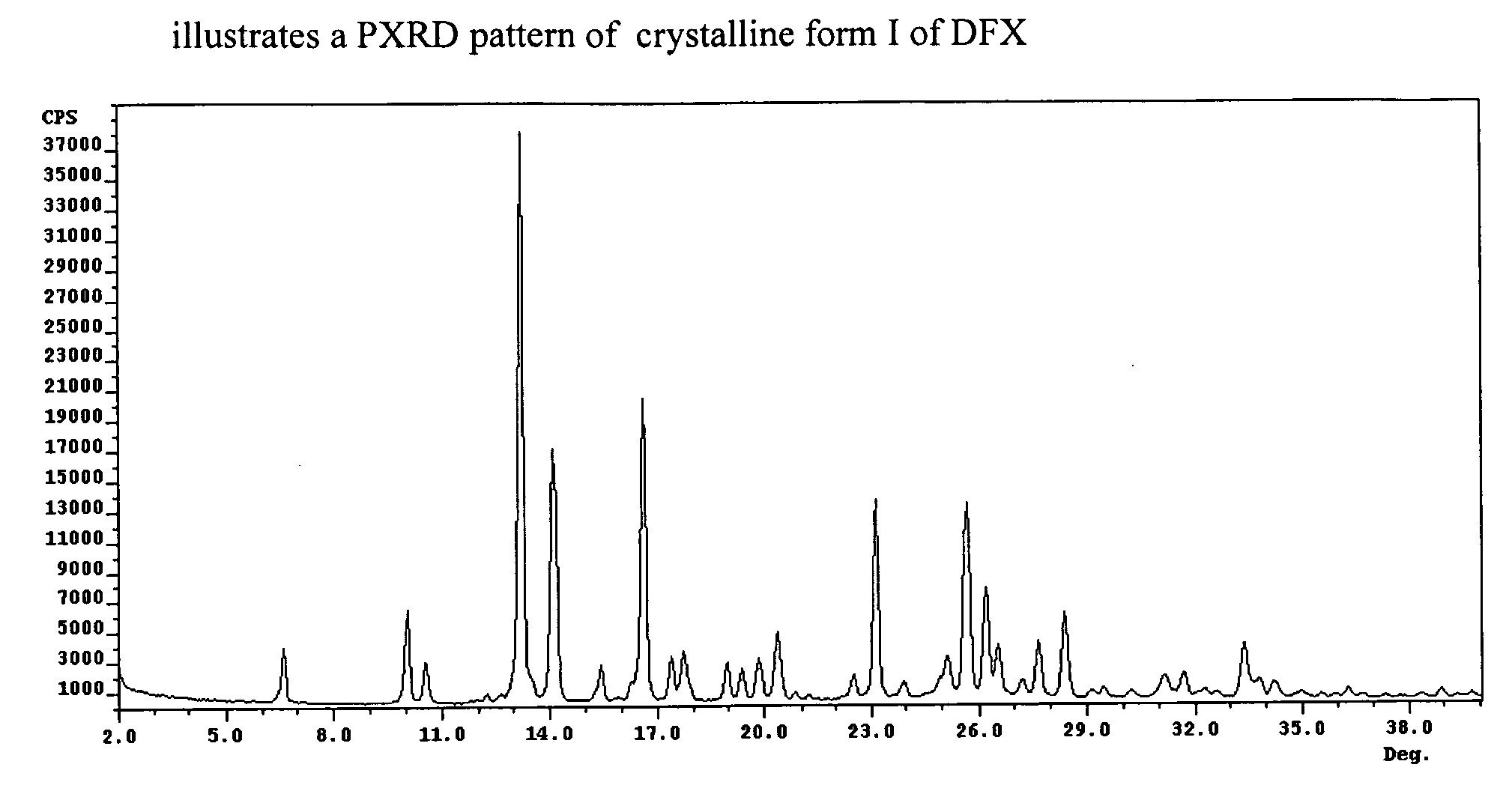
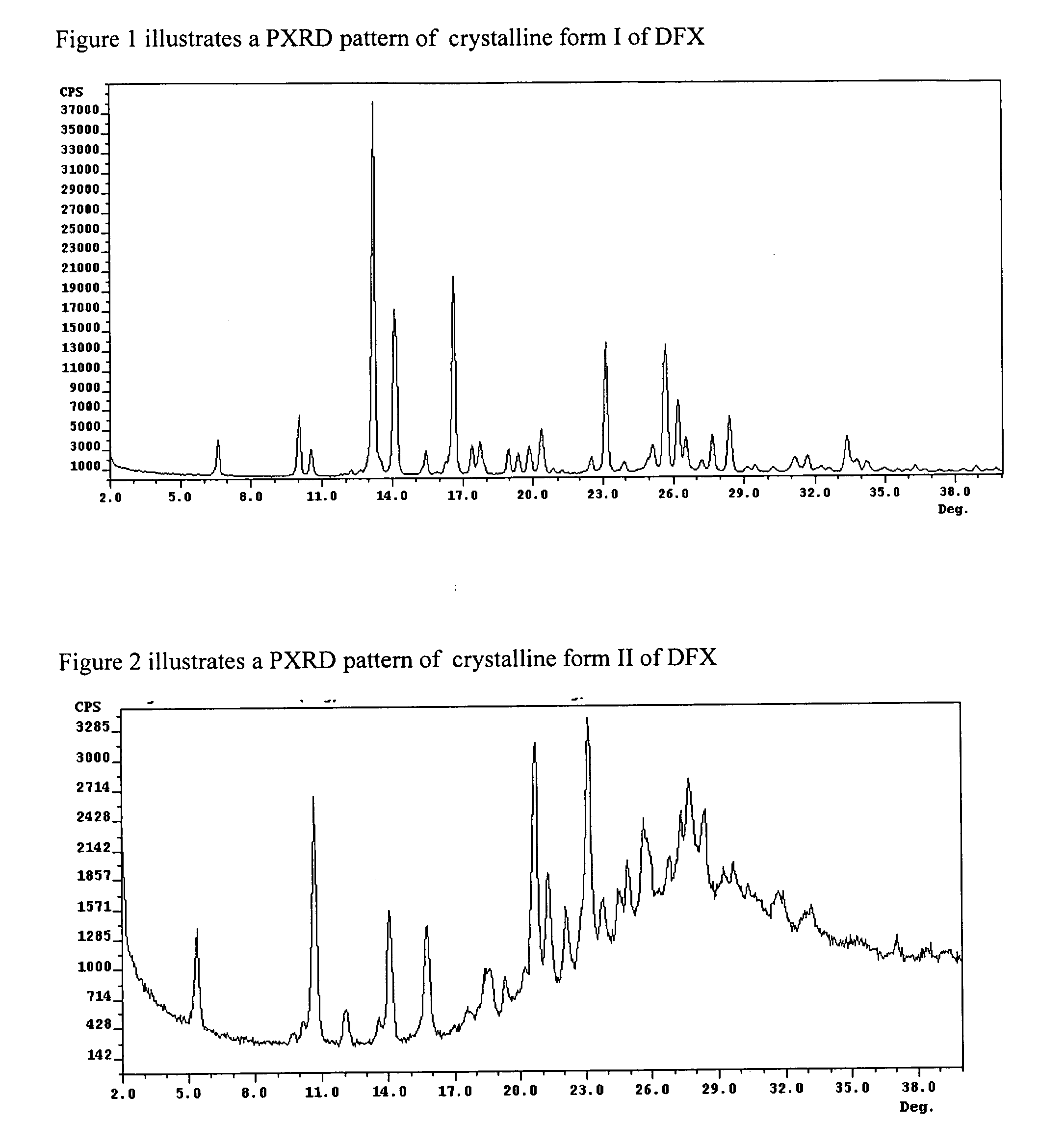
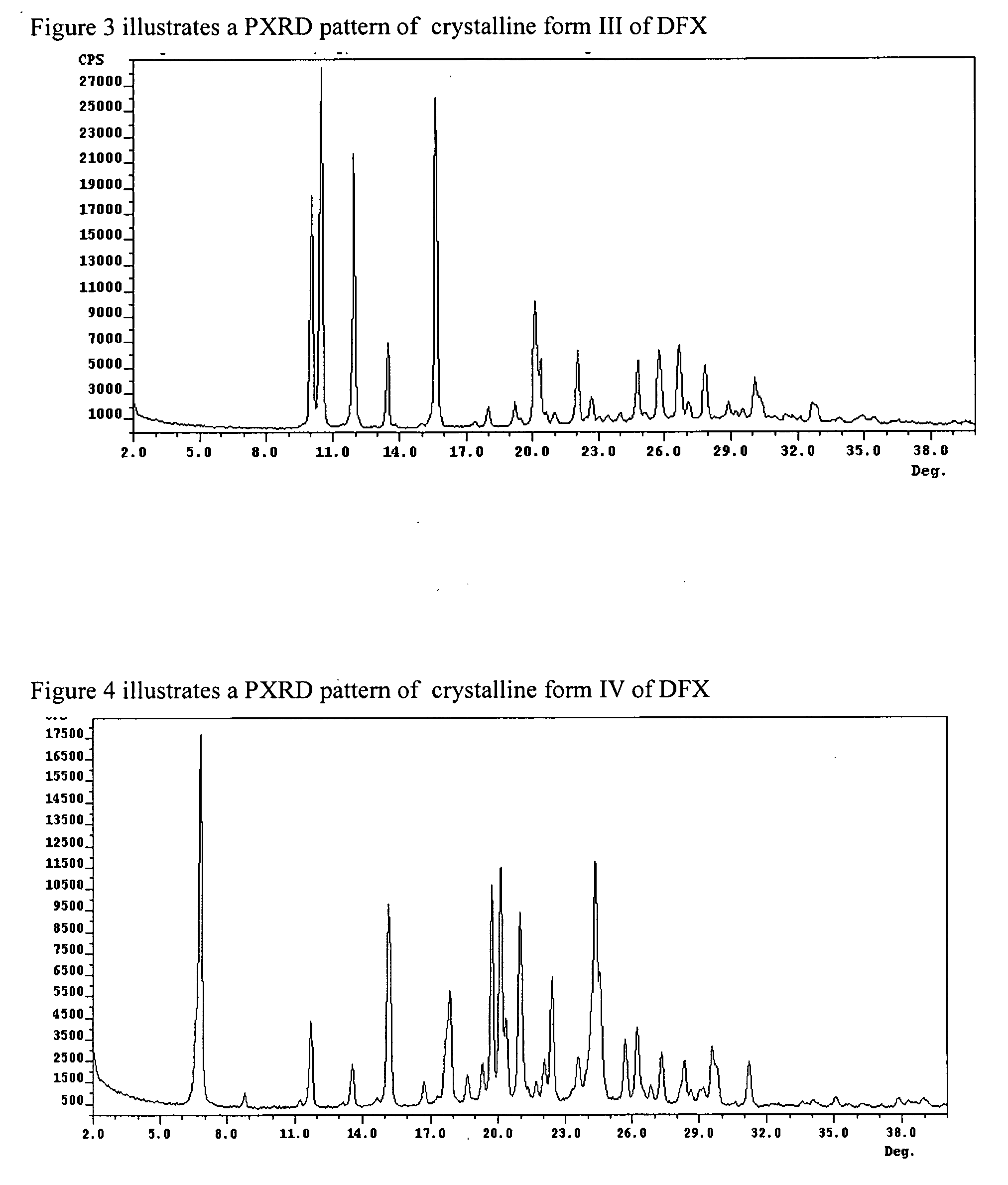
Crystalline forms of Deferasirox
The present invention provides novel crystalline forms of deferasirox, methods for their production, and methods for conversion of the novel forms to the known crystalline form I.
Owner:TEVA PHARM USA INC
HIF-1 Modulator Paint Formulation and Uses Thereof
ActiveUS20150196512A1Improve permeabilityGood sustained releaseBiocidePowder deliveryIron ChelatorMedicine
Formulations and methods are provided for improving the function, i.e. clinical outcome, of solid organ transplants. Lung transplantation is of particular interest. In the methods of the invention, a nanoparticle formulation comprising an effective dose of an iron chelator active agent in nanoparticle form, including without limitation, deferoxamine (DFO), deferasirox (DFX), and deferiprone (DFP), etc. suspended in a carrier compatible with the tissue of interest, is topically applied to the surface of tissues at the site of anastomosis. The nanoparticles are comprised of the active agent and a pharmaceutically acceptable stabilizer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +2
Low Dose Pharmaceutical Composition
InactiveUS20160120847A1Eliminate side effectsImprove bioavailabilityBiocidePowder deliveryDeferasiroxDeferiprone
This invention provides a low dose pharmaceutical composition comprising deferasirox or a pharmaceutically acceptable derivative thereof and one or more pharmaceutically acceptable excipients. A unit dose of the pharmaceutical composition comprises from about 50 mg to about 100 mg of deferasirox, from about 150 mg to about 200 mg of deferasirox or from about 260 mg to about 350 mg of deferasirox. The pharmaceutical composition of the present invention, wherein the pharmaceutical composition comprises deferasirox, may be used to treat chronic iron overload or to treat lead toxicity. The pharmaceutical composition of the present invention, wherein the pharmaceutical composition comprises deferasirox and deferiprone, may be used to treat lead toxicity. This invention also provides a process for preparing the low dose pharmaceutical composition, the process comprising: dissolving or adsorbing or blending deferasirox and at least one excipient to produce a dispersion of deferasirox; and processing the dispersion to produce a desired dosage form.
Owner:CIPLA LTD
Processes for the preparation of deferasirox, and deferasirox polymorphs
ActiveUS20120245361A1Improve bioavailabilityMaintain good propertiesSilicon organic compoundsMetabolism disorderIron ChelatorPharmaceutical drug
The present invention relates to processes for the preparation of deferasirox, an oral iron chelator developed to treat iron overload due to e.g. multiple blood transfusions. The present invention further provides novel deferasirox pseudopolymorphs and a novel amorphous form of deferasirox, processes for their preparation, as well as pharmaceutical compositions comprising same, and use thereof in treating iron overload.
Owner:MAPI PHARMA
Preparation method of deferasirox and intermediate compound of deferasirox
ActiveCN103396373AGentle preparation processThe preparation process has mild reaction conditionsOrganic chemistryChlorideDeferasirox
The invention belongs to the field of pharmaceutical chemicals, provides a preparation method of deferasirox which is an iron-overloaded medicament and particularly provides a method for preparing deferasirox mainly from salicyloyl chloride, o-benzyloxy cyanophenyl, p-hydrazinobenzoic acid (or hydrochlorides thereof) under mild conditions. The method for preparing the deferasirox is mild in condition and simple to operate; the deferasirox product is high in purity.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Deferasirox granules and preparation method thereof
InactiveCN103735519AEasy doseEasy to takeOrganic active ingredientsPharmaceutical non-active ingredientsPatient complianceAdhesive
The invention provides deferasirox granules and a preparation method thereof, and belongs to the field of medicinal preparations. The deferasirox granules comprise a main component and auxiliary components, wherein deferasirox serves as a main component; the auxiliary components include a filling agent, a disintegrating agent, an adhesive, a surface active agent, a corrigent, etc. The deferasirox granules can be quickly dissolved in water to form uniform suspension, taste is great, and the deferasirox granules are convenient to carry, accurate in quantifying, easy to divide in dose, and high in bioavailability, etc.; the deferasirox granules can improve patient compliance and are particularly suitable for children.
Owner:无锡万全医药技术有限公司
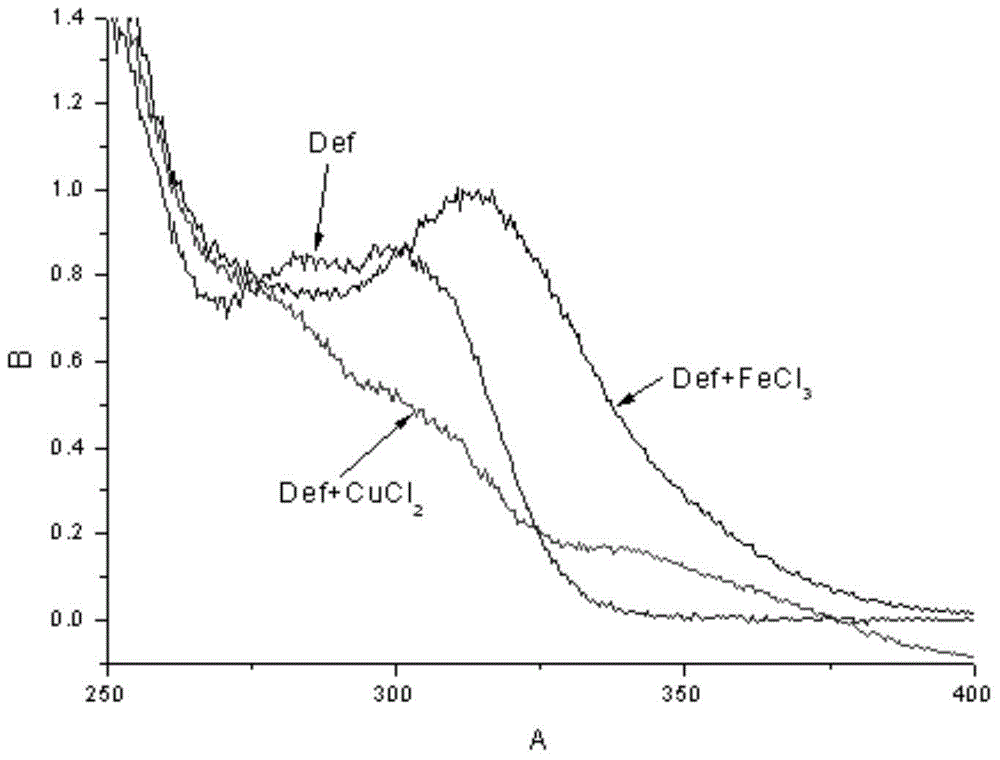
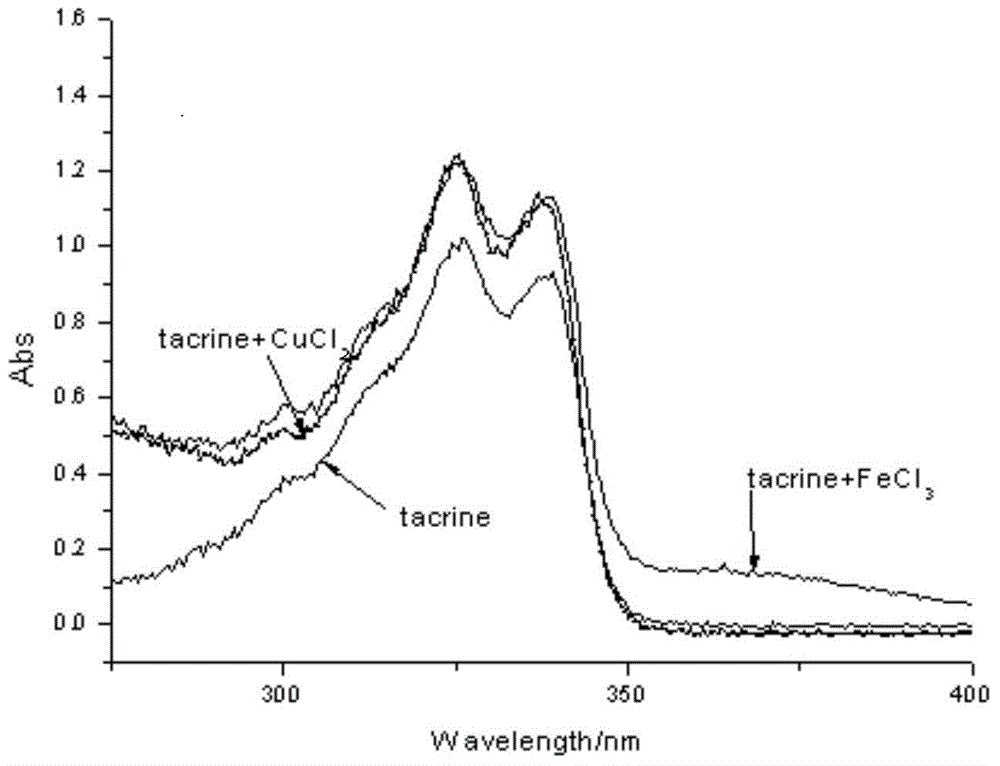
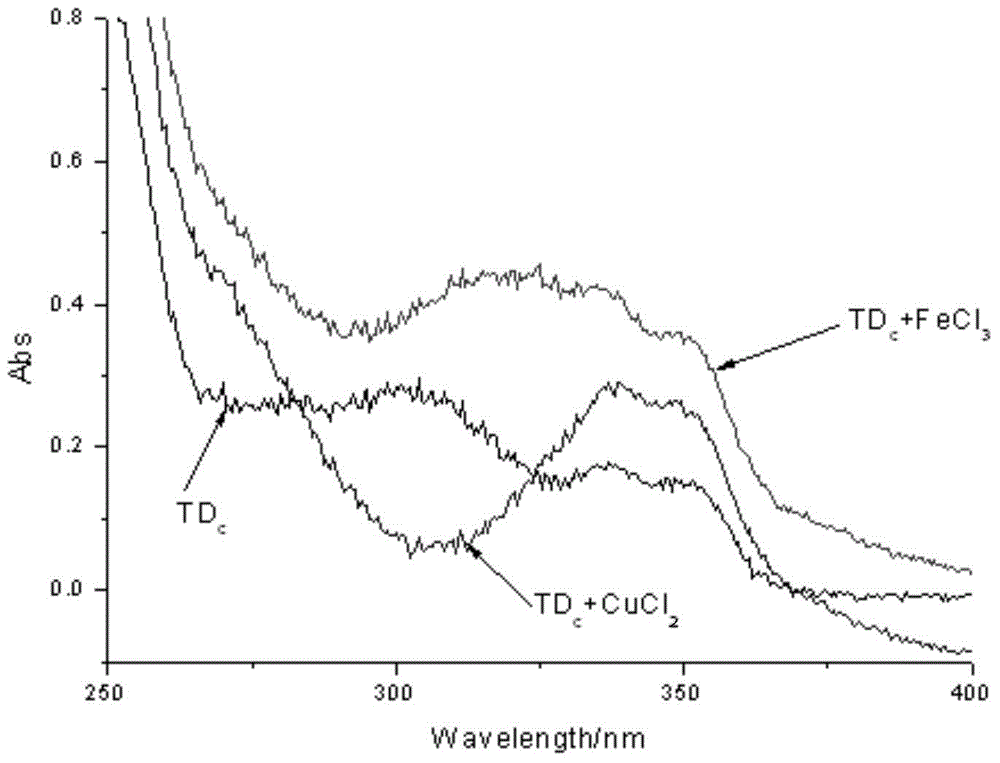
Deferasirox-tacrine metal ion chelating agent and pharmaceutical use thereof
InactiveCN104817538AOvercoming the shortcomings of acting on a single targetImprove utilization efficiencyNervous disorderOrganic chemistryDiseaseCarbon chain
The invention provides a deferasirox-tacrine metal ion chelating agent, the structural formula of which is shown as formula I. The invention also provides a preparation method of the deferasirox-tacrine metal ion chelating agent and application of the chelating agent in preparation of drugs treating Alzheimer's disease. According to the invention, deferasirox and tacrine are connected together through carbon chains of different lengths, the metal-chelating properties and antioxidant properties of deferasirox are reserved, and the connected tacrine matrix retains the original acetylcholine esterase inhibitory activity. The obtained novel ion chelating agent has multiple functions and can act on multiple target points. (formula I).
Owner:SOUTHEAST UNIV
Formulations of deferasirox and methods of making the same
ActiveUS20170296514A1Improve bioavailabilityOrganic active ingredientsPowder deliveryMedicineDeferasirox
The disclosure provides for improved pharmaceutical compositions containing deferasirox (DFX) and methods of manufacturing the same. In particular, the compositions are prepared using thermokinetic compounding and provide improved properties as well as more efficient methods of manufacture.
Owner:AUSTINPX LLC
Formulations of deferasirox and methods of making the same
ActiveUS10258608B2Improve bioavailabilityOrganic active ingredientsPowder deliveryMedicineDeferasirox
The disclosure provides for improved pharmaceutical compositions containing deferasirox (DFX) and methods of manufacturing the same. In particular, the compositions are prepared using thermokinetic compounding and provide improved properties as well as more efficient methods of manufacture.
Owner:AUSTINPX LLC
Preparation method of deferasirox and intermediate compound of deferasirox
ActiveCN103396373BGentle preparation processThe preparation process has mild reaction conditionsOrganic chemistryChlorideDeferasirox
The invention belongs to the field of pharmaceutical chemicals, provides a preparation method of deferasirox which is an iron-overloaded medicament and particularly provides a method for preparing deferasirox mainly from salicyloyl chloride, o-benzyloxy cyanophenyl, p-hydrazinobenzoic acid (or hydrochlorides thereof) under mild conditions. The method for preparing the deferasirox is mild in condition and simple to operate; the deferasirox product is high in purity.
Owner:NANJING HAIRUN PHARM CO LTD +1
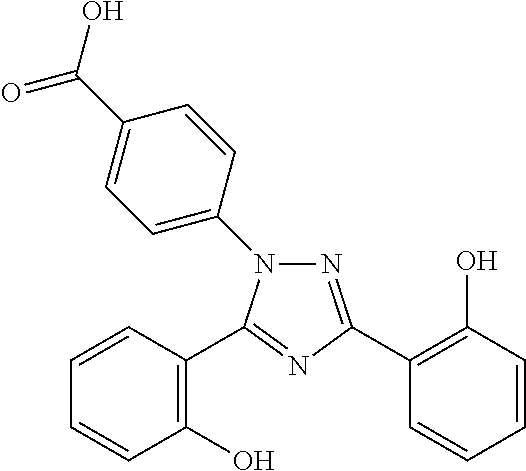
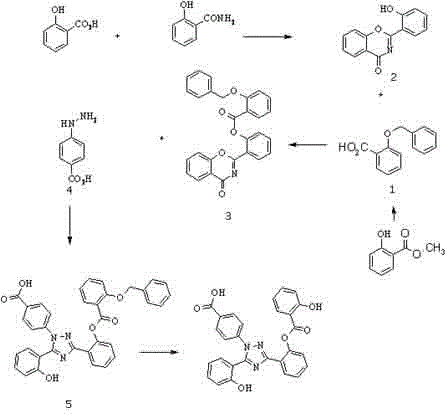
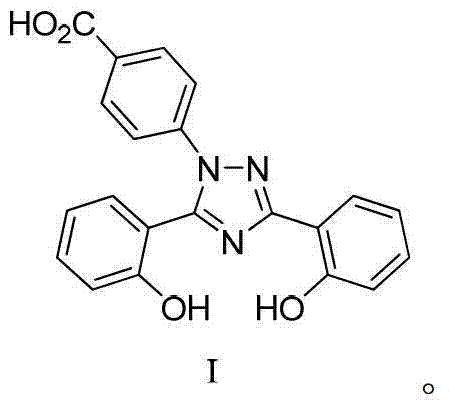
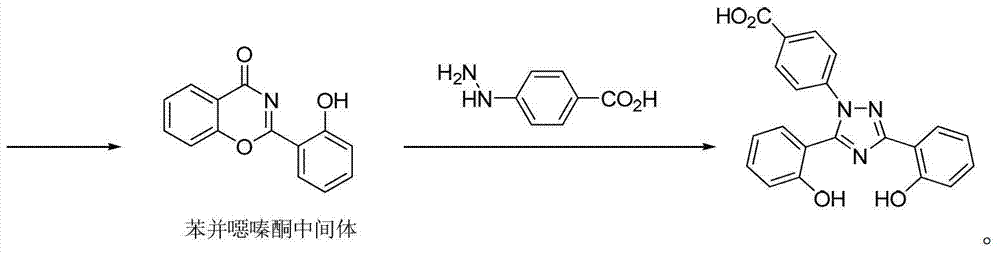
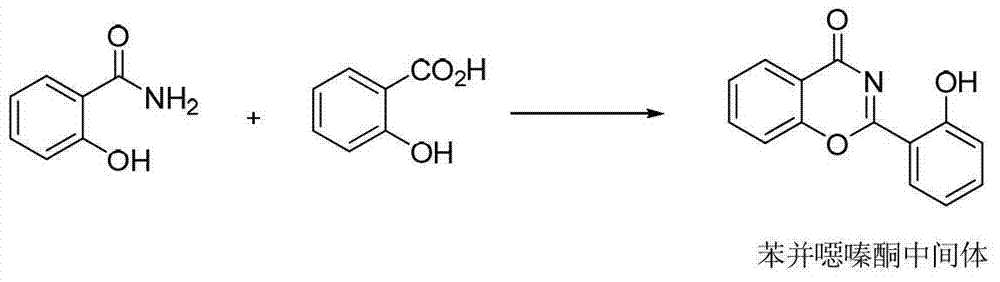
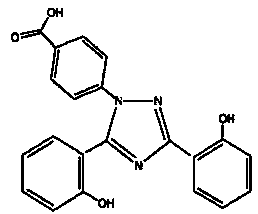
Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid
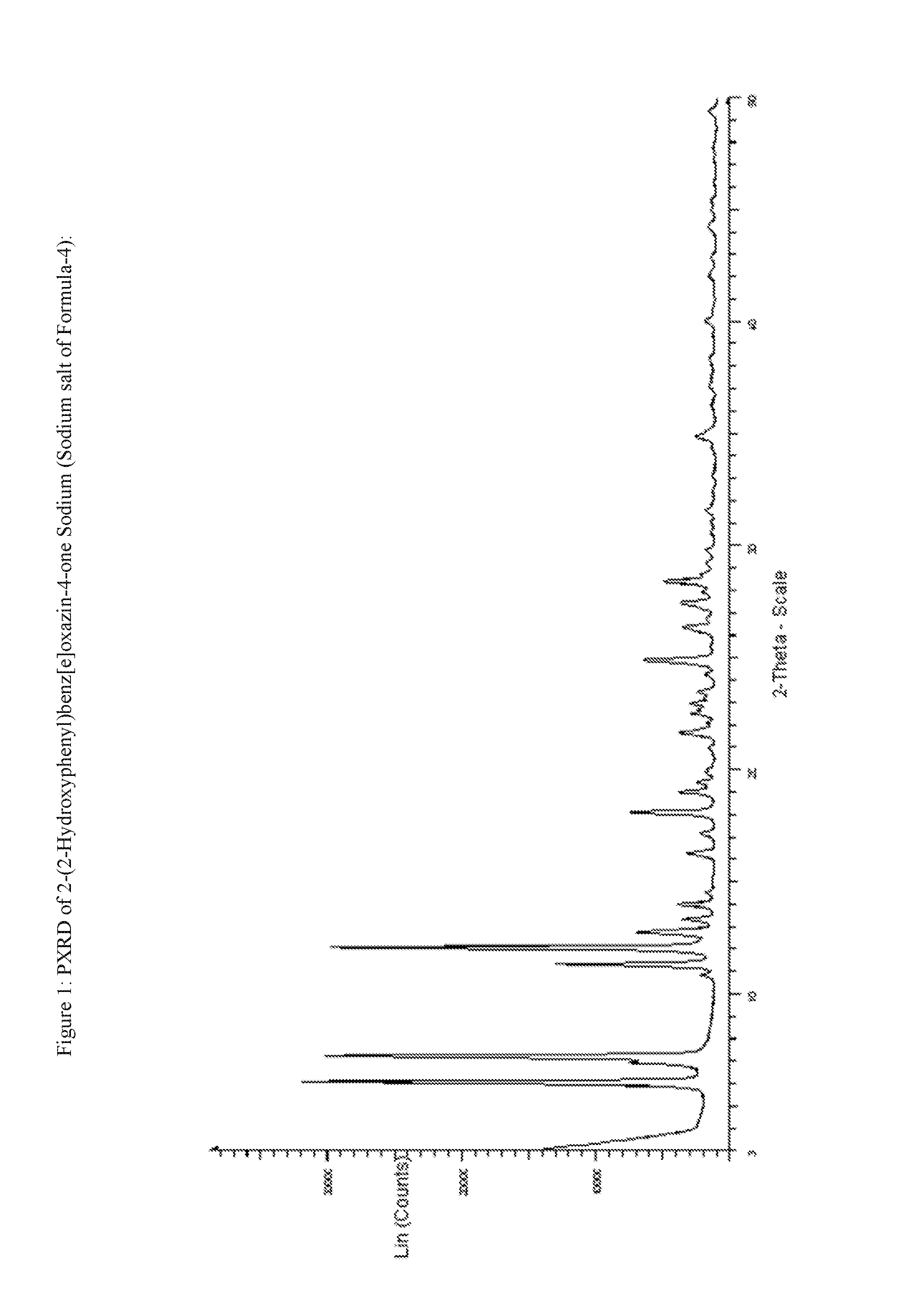
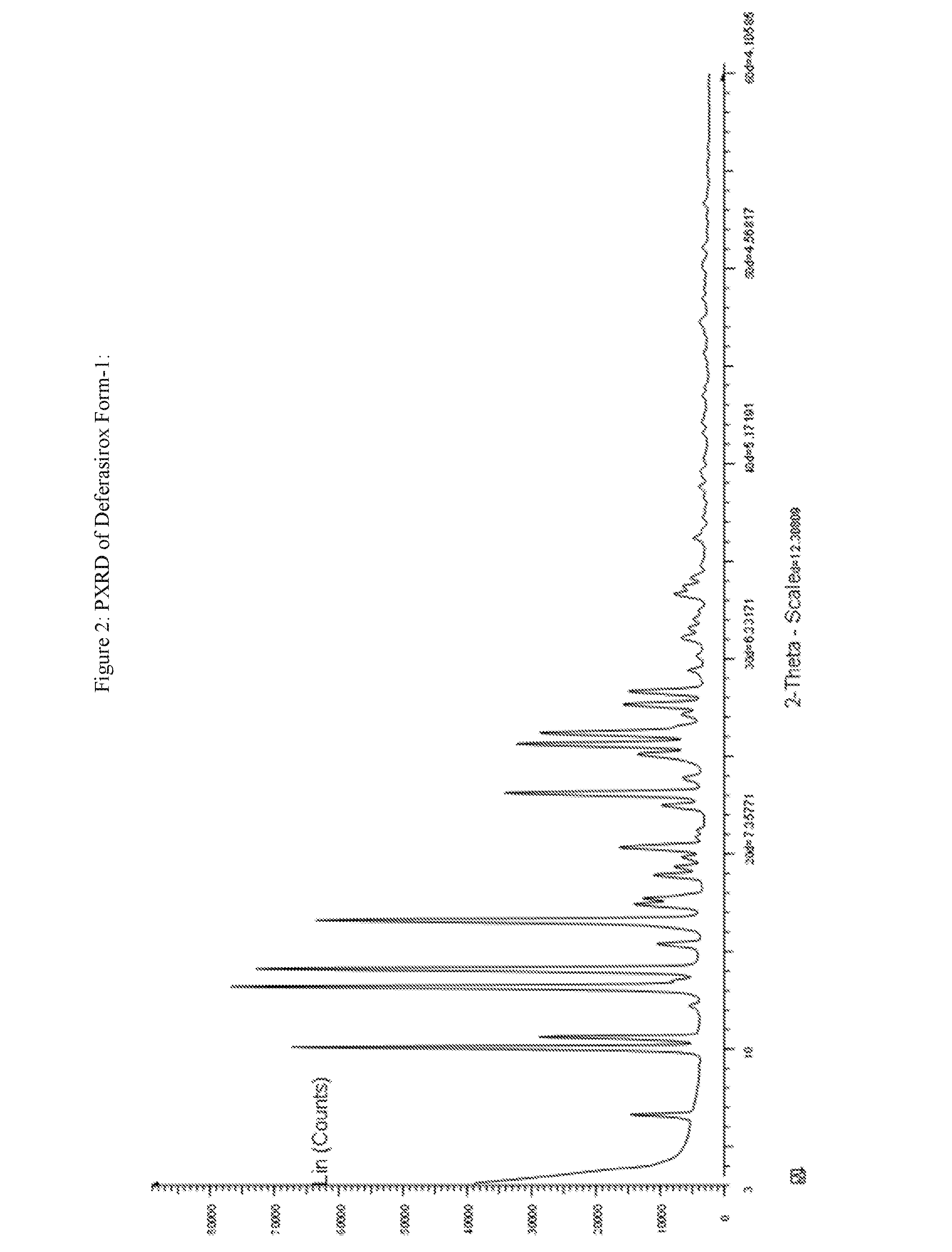
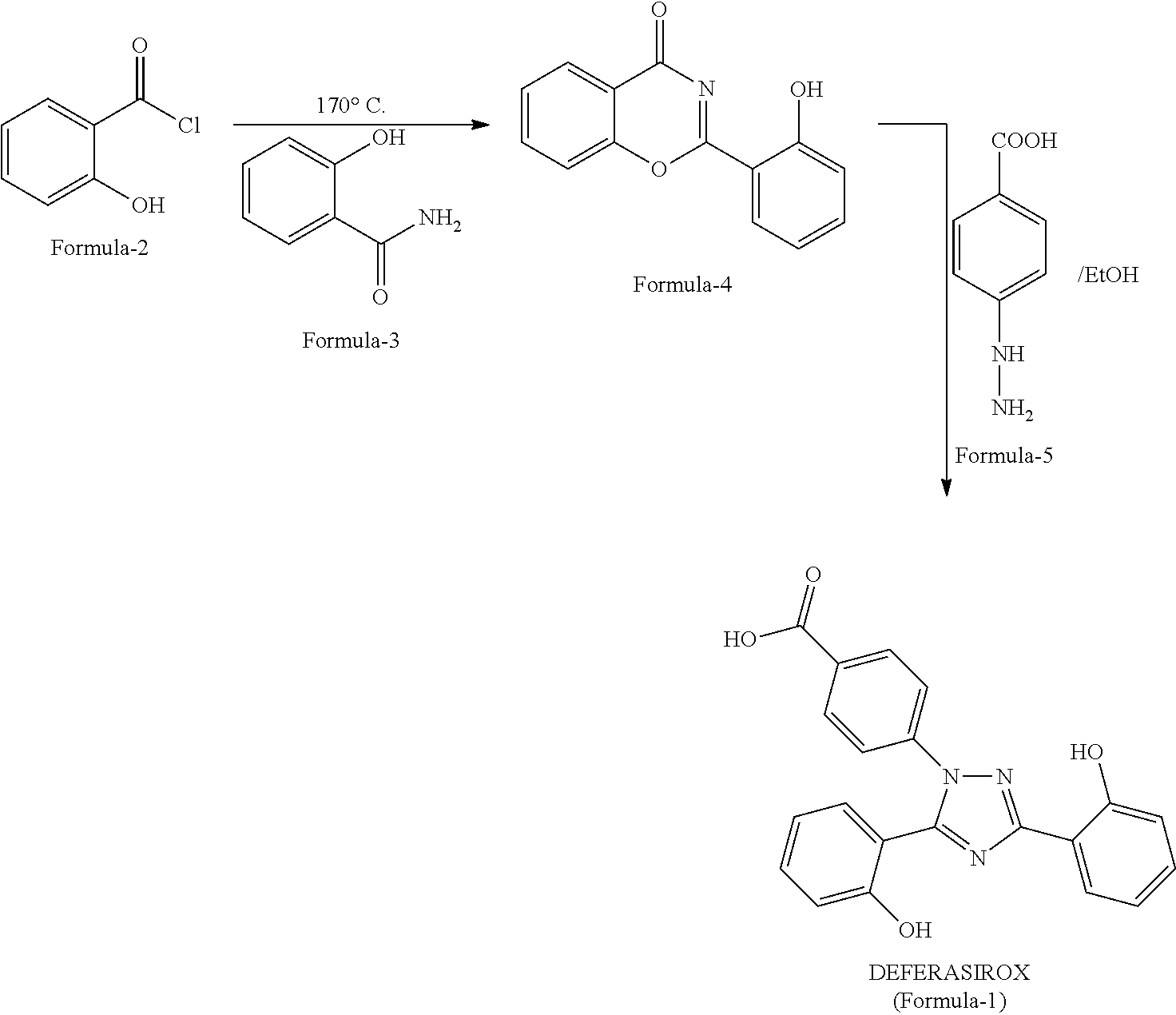
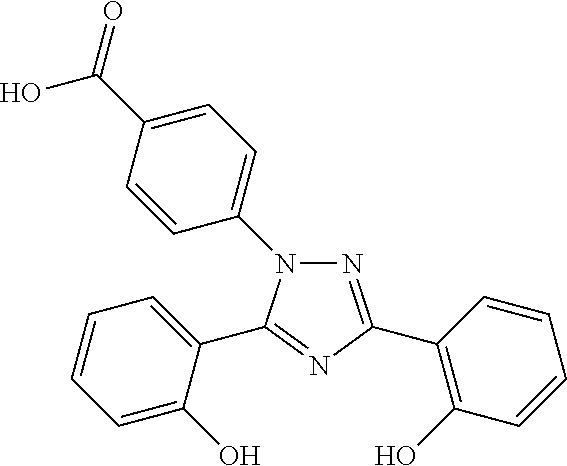
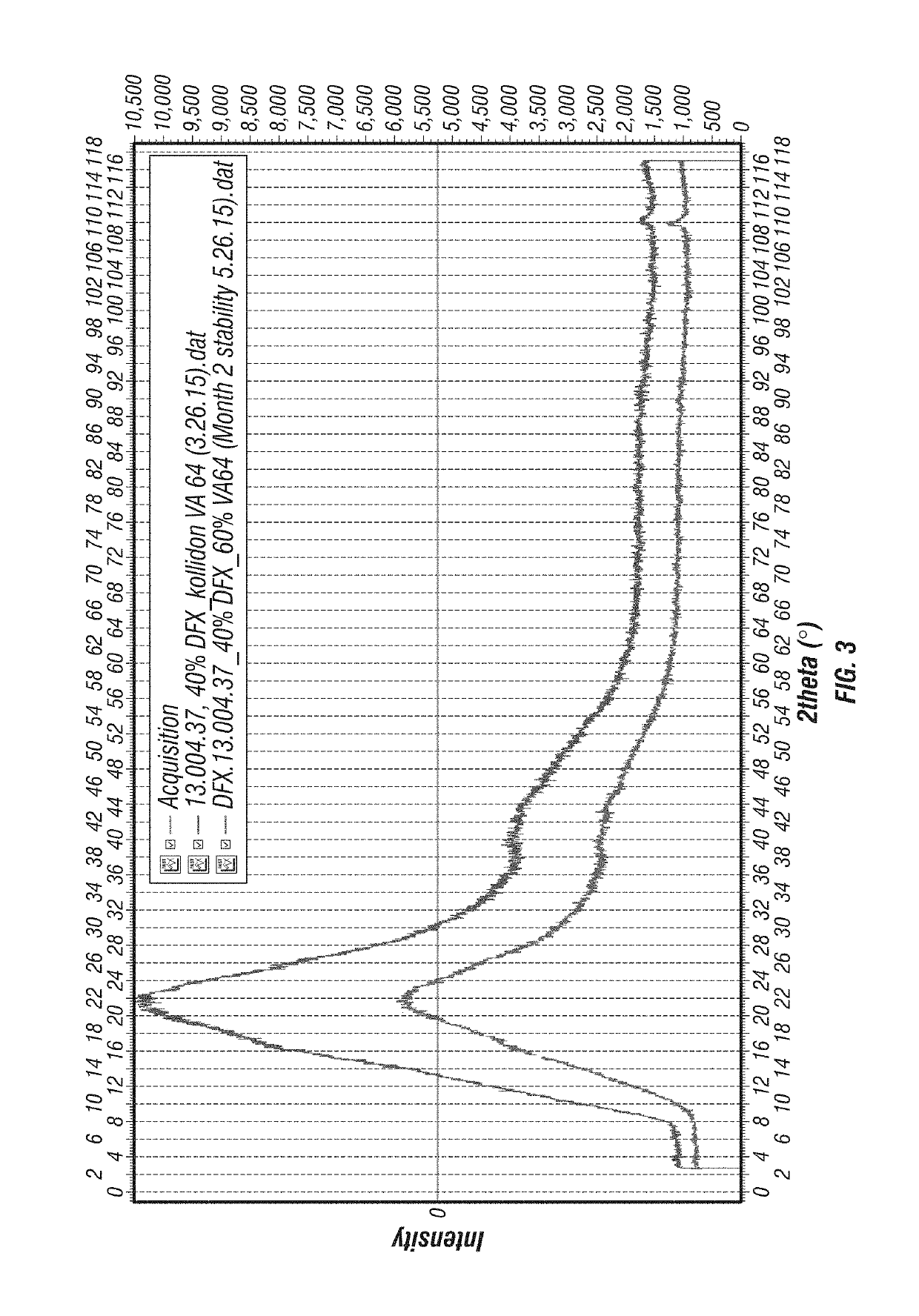
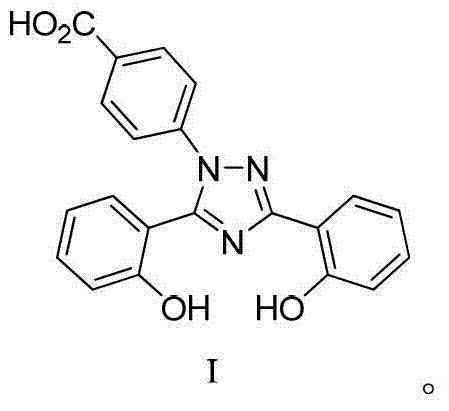
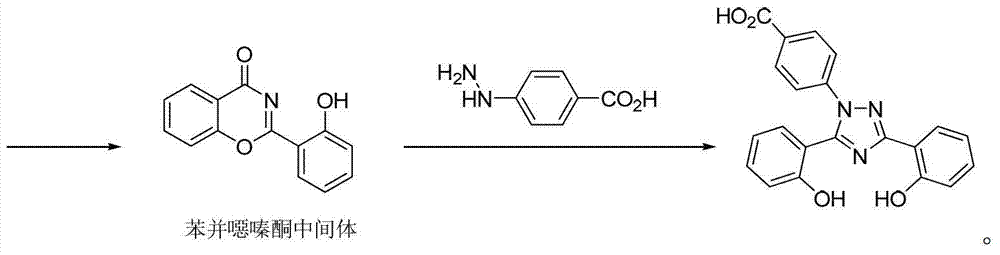
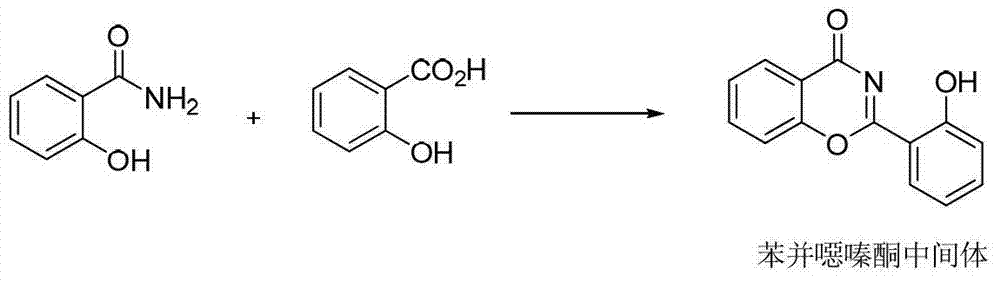
InactiveUS20130338356A1Low yieldHigh yieldRubidium/caesium/francium compoundsAlkali metal oxides/hydroxidesBenzoic acidSalicylic acid
The invention provides a novel process for the synthesis of 2-(2-hydroxyphenyl)-benz[1,3]oxazin-4-one, the process comprising of reacting the salicylic acid with salicylamide in the presence of p-toluenesulfonyl chloride, base and solvent. The use of 2-(2-hydroxyphenyl)-benz[1,3]oxazin-4-one in the preparation of Deferasirox is also disclosed in the invention.
Owner:DAVULURI RAMAMOHAN RAO
Process for the preparation of deferasirox
Present disclosure discloses the commercially viable process for the preparation of Deferasirox and its polymorph with. Disclosed process involves the preparation of Deferasirox via metal salt of the corresponding intermediate and deferasirox metal salt.
Owner:BIOCON LTD
Use of Deferiprone and Methods to Treat and/or Prevent Friedreich Ataxia Resulting from Intracellular Mishandling of Iron
A therapeutically effective amount of deferiprone or deferasirox or physiologically acceptable salts thereof for the prevention, stabilization, treatment, or reversal of iron-induced FRDA disease in patients resulting from mitochondrial iron-induced damage to preferentially reduce the iron stores in the mitochondria. Also for the treatment of other conditions affecting the brain where a key element in the generation of the resultant pathology is the intracellular mishandling of iron.
Owner:MUNNICH ARNOLD +2
Immediate release pharmaceutical composition of iron chelating agents
ActiveUS10888519B2High drug loadingImprovement ingredientsOrganic active ingredientsBlood disorderPharmaceutical drugDeferasirox
The present invention relates to a stable, immediate release solid oral pharmaceutical compositions comprising iron chelating agents like Deferasirox and at least one pharmaceutical acceptable excipient wherein the composition is free of glidant. Prior art discloses various technical challenges and suggest restrictive and complex solutions for the development of immediate release dosage forms of Deferasirox such as utilizing a large number of excipients or non-conventional formulation techniques. The glidant free immediate release solid oral pharmaceutical composition of Deferasirox, prepared as per present invention exhibited desirable technical attributes like pharmaceutical stability, flow properties and comparable dissolution, bioequivalence against reference listed drug.
Owner:JUBILANT GENERICS
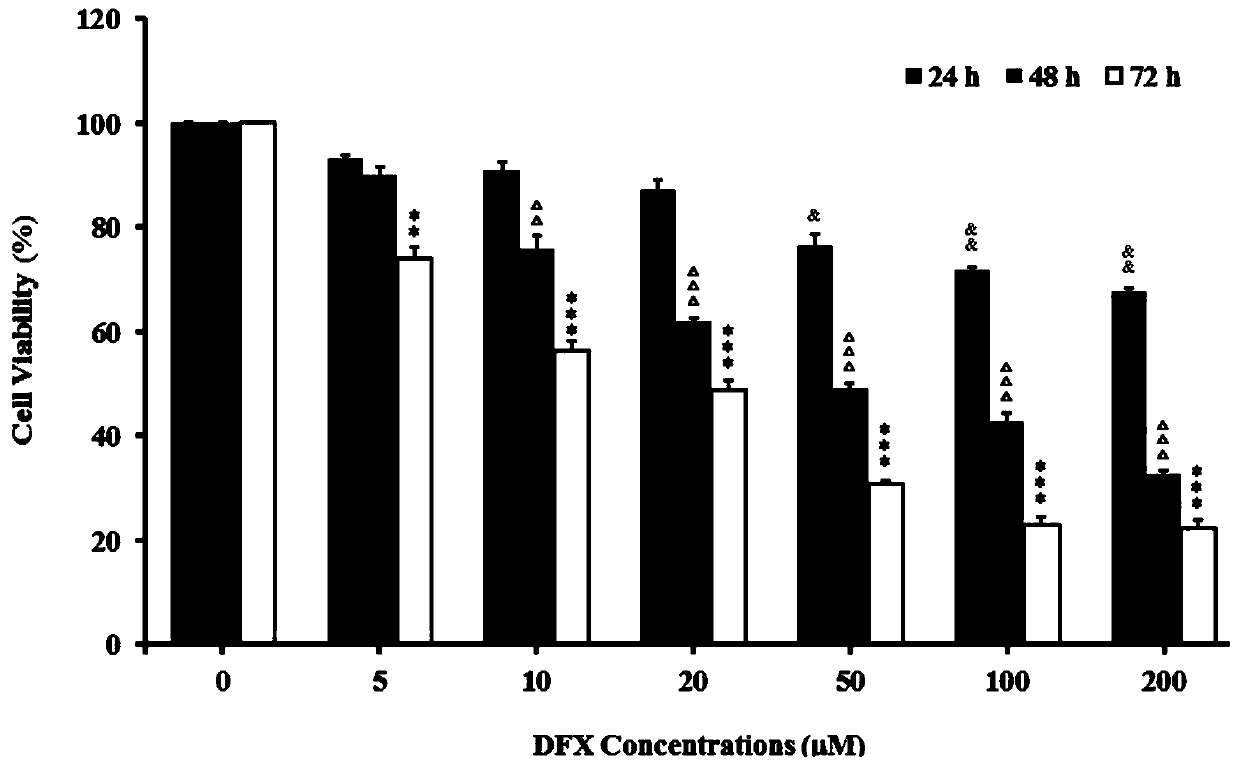
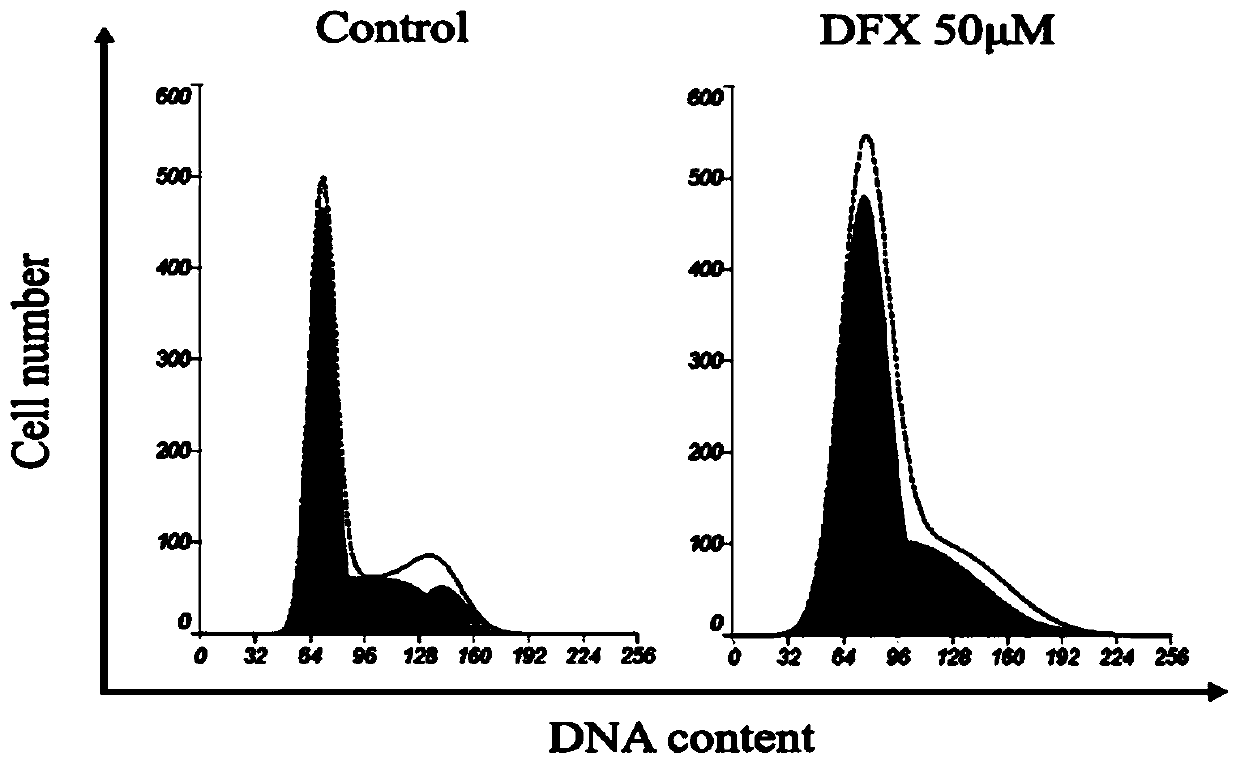
Application of iron chelator Deferasirox (DFX) in drug for treating cervical cancer
PendingCN109745314AStrong growth inhibitory effectEasy to useOrganic active ingredientsAntineoplastic agentsIron ChelatorOxidative stress
The invention discloses application of an iron chelator Deferasirox (DFX) in a drug for treating cervical cancer. The DFX is used for inhibiting the levels of a labile iron pool (LIP) and oxidative stress (ROS) in cervical cancer cells to significantly reduce the iron content in tumor tissue, and inhibiting the proliferation and division of the cervical cancer cells by retarding a fission cycle ofthe cervical cancer cells, thereby inhibiting the tumor growth.
Owner:HEBEI NORMAL UNIV
Preparation method of deferasirox derivative
ActiveCN103554040AEasy to operateImprove production efficiencyOrganic chemistryBlood disorderReaction temperatureCombinatorial chemistry
The invention discloses a preparation method of a deferasirox derivative. The preparation process comprising optimal reactant usage amount, reaction temperatures, reaction time, reaction steps and the like is determined via a large quantity of experimental screening. The whole preparation process is strong in maneuverability, high in preparation efficiency and low in production cost; and especially, the total yield can reach more than 30%. Compared with the prior art that the total yield is only 14% or so, the preparation process disclosed by the invention has a good technical effect. In addition, the whole preparation process is convenient for purification, thereby overcoming the technical defect of complicated separation and purification in the prior art and having the relatively high production efficiency. Thus, the industrial mass production can be realized.
Owner:TLC NANJING PHARMA RANDD CO LTD
Preparation method and pharmaceutical preparation of deferasirox solid dispersion
ActiveCN105853367AHigh dissolution rateImprove dispersionOrganic active ingredientsPowder deliveryOrganic solventMethyl cellulose
The invention provides a preparation method and a pharmaceutical preparation of deferasirox solid dispersion. The preparation method includes uniformly mixing deferasirox with pharmaceutically acceptable carriers, heating the mixture to melt, cooling and grinding to obtain the deferasirox solid dispersion, wherein the pharmaceutically acceptable carriers are selected from povidone, hydroxypropyl methyl cellulose and copovidone. The invention further discloses a deferasirox tablet containing the deferasirox solid dispersion. The preparation method free of organic solvents is beneficial to lowering safety risks and environmental protection pressure, reduces production cost and is conducive to commercial production of products.
Owner:安士制药(中山)有限公司
Processes for the preparation of deferasirox, and deferasirox polymorphs
ActiveUS8772503B2Improve bioavailabilityMaintain good propertiesSilicon organic compoundsMetabolism disorderIron ChelatorPharmaceutical drug
The present invention relates to processes for the preparation of deferasirox, an oral iron chelator developed to treat iron overload due to e.g. multiple blood transfusions. The present invention further provides novel deferasirox pseudopolymorphs and a novel amorphous form of deferasirox, processes for their preparation, as well as pharmaceutical compositions comprising same, and use thereof in treating iron overload.
Owner:MAPI PHARMA
Polymorphic forms of deferasirox (icl670a)
The invention relates to crystalline forms of -[3,5-bis(2-hydroxyphenyl)-[1,2,4]triazol-1-yl]benzoic acid and to its amorphous form, to processes for the preparation thereof, to compositions containing the same and their uses for the manufacture of a medicament for the treatment of the human body.
Owner:NOVARTIS AG
Pharmaceutical Composition Comprising Deferasirox
InactiveUS20160324831A1Increase the areaImprove solubilityPowder deliveryOrganic active ingredientsSolubilityDeferasirox
The present invention relates to a pharmaceutical composition comprising deferasirox, a process for preparing such pharmaceutical composition, and its use in the treatment of chronic iron overload. The pharmaceutical composition comprises nanosized deferasirox having improved surface area and solubility. It also relates to a method for treatment of chronic iron overload which comprises administering a pharmaceutical composition comprising nanosized deferasirox.
Owner:CIPLA LTD
Oral formulations of deferasirox
InactiveUS20180071220A1Dissolve fastIncrease exposureOrganic active ingredientsCoatingsNeutral phFast release
Orally administerable deferasirox formulations are disclosed having reduced release under gastric conditions and fast release at near neutral pH or at neutral pH.
Owner:GHOSH INDRAJIT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid](https://images-eureka.patsnap.com/patent_img/6f5374f4-1e12-44fc-a439-c9e063d03fee/US20130338356A1-20131219-C00001.png)
![Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid](https://images-eureka.patsnap.com/patent_img/6f5374f4-1e12-44fc-a439-c9e063d03fee/US20130338356A1-20131219-C00002.png)
![Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid Process for the preparation, of 2-(2-hydroxyphenyl)-benz [1, 3] oxazin-4-one and its use for preparation of 4-[3, 5-bis (2-hydroxyphenyl)-lH-l , 2, 4-triazolTl-yl] benzoic acid](https://images-eureka.patsnap.com/patent_img/6f5374f4-1e12-44fc-a439-c9e063d03fee/US20130338356A1-20131219-C00003.png)