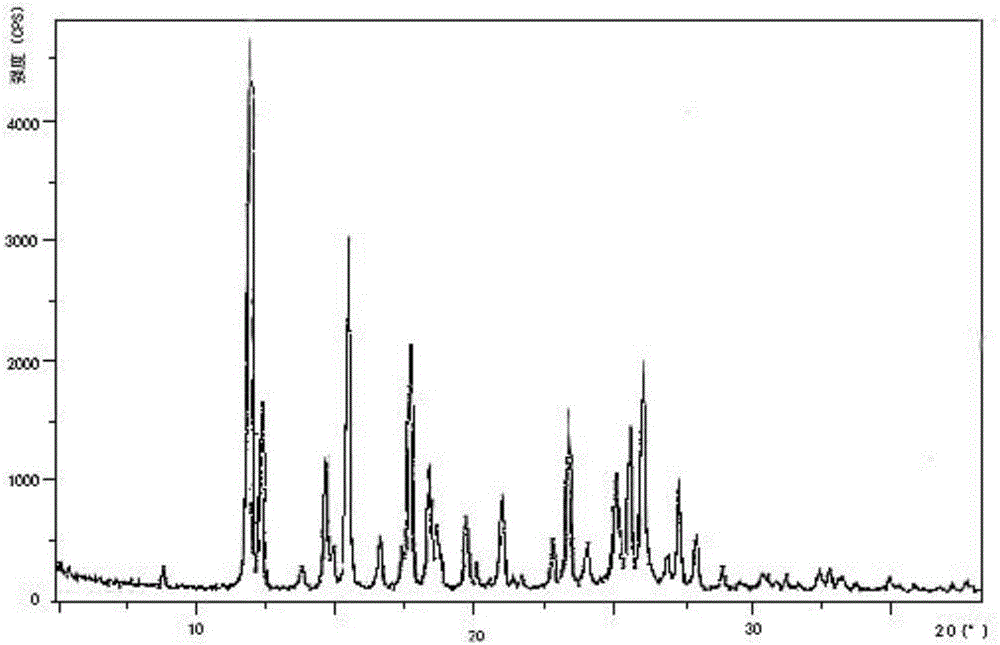
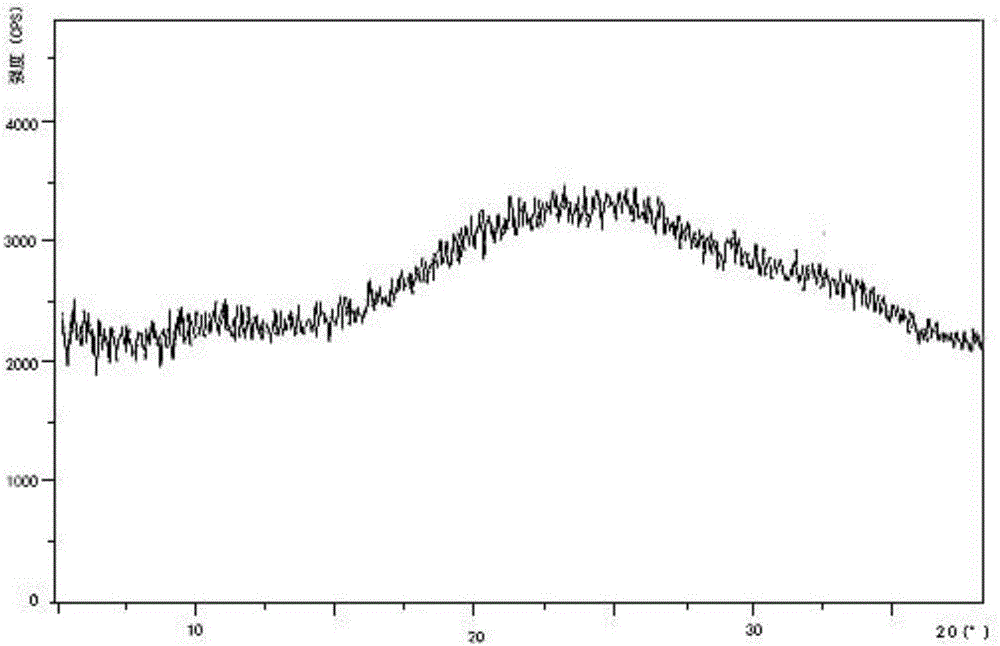
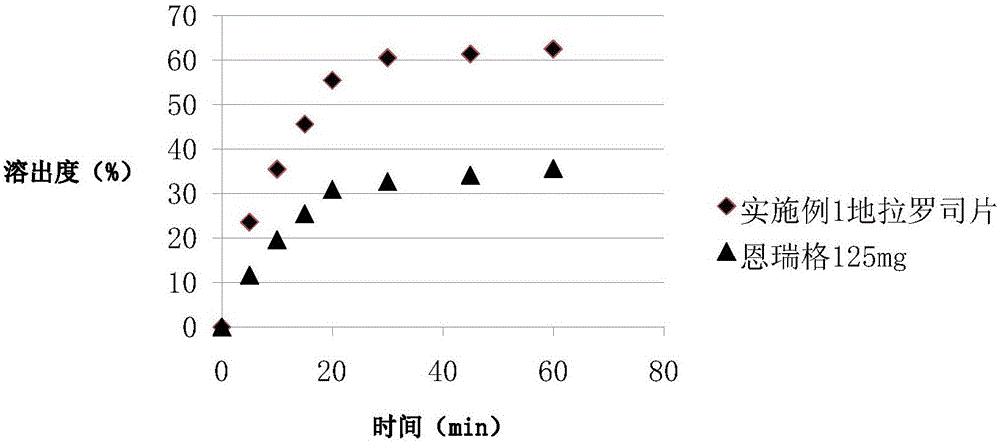
Preparation method and pharmaceutical preparation of deferasirox solid dispersion
A technology of solid dispersion and deferasirox, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as adverse reactions, short half-life, and restrictions on widespread use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Mix deferasirox and povidone uniformly, and extrude them with Thermo Fisher Pharma 11 twin-screw hot melt extruder. The screw speed is 10-30 rpm. The heating temperature is controlled as shown in the table below. After the extrudate is cooled, use FITZ-MILL The hammer mill is crushed into particles with a screen aperture of 1.0mm; the particles are mixed evenly with crospovidone, lactose, microcrystalline cellulose, colloidal silicon dioxide, magnesium stearate, and sodium lauryl sulfonate. Compressed into tablets.
[0039]
Embodiment 2
[0042] Mix deferasirox and povidone uniformly, and extrude them with Thermo Fisher Pharma 11 twin-screw hot melt extruder. The screw speed is 10-30 rpm. The heating temperature is controlled as follows. After the extrudate is cooled, use FITZ-MILL The hammer mill is crushed into particles with a screen aperture of 1.0mm; the particles are mixed evenly with crospovidone, lactose, microcrystalline cellulose, colloidal silicon dioxide, magnesium stearate, and sodium lauryl sulfonate. Compressed into tablets.
[0043]
Embodiment 3
[0046] Mix deferasirox and povidone uniformly, and extrude them with Thermo Fisher Pharma 11 twin-screw hot melt extruder. The screw speed is 10-30 rpm. The heating temperature is controlled as shown in the table below. After the extrudate is cooled, use FITZ-MILL The hammer mill is crushed into particles with a screen aperture of 1.0mm; the particles are mixed evenly with crospovidone, lactose, microcrystalline cellulose, colloidal silicon dioxide, magnesium stearate, and sodium lauryl sulfonate. Compressed into tablets.
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com