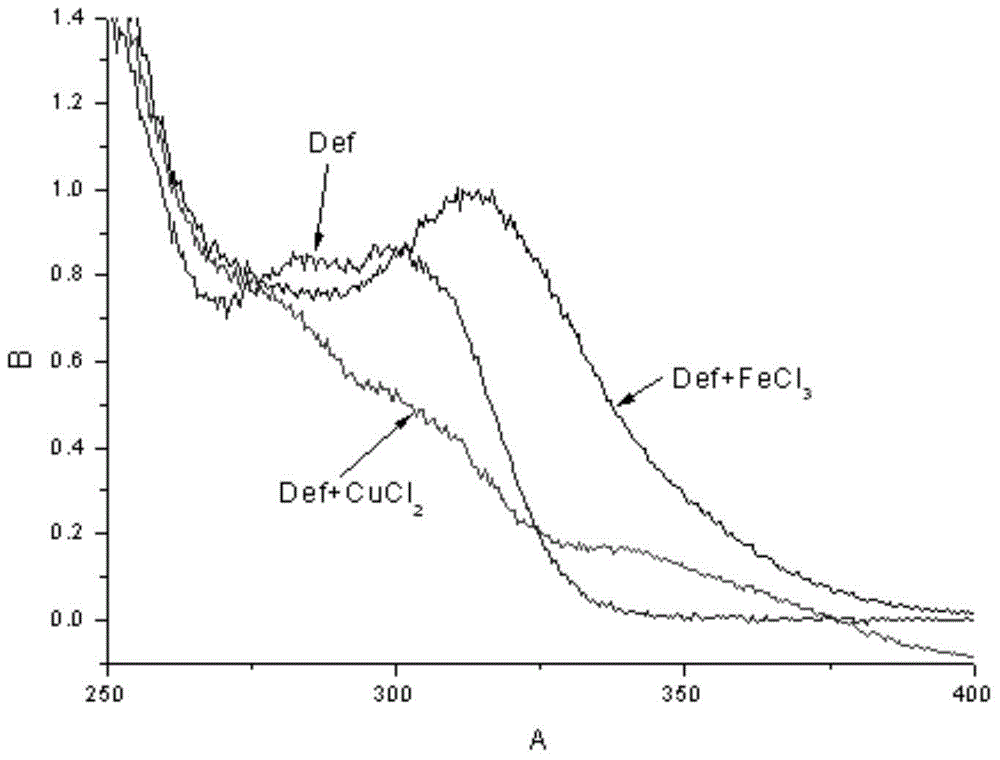
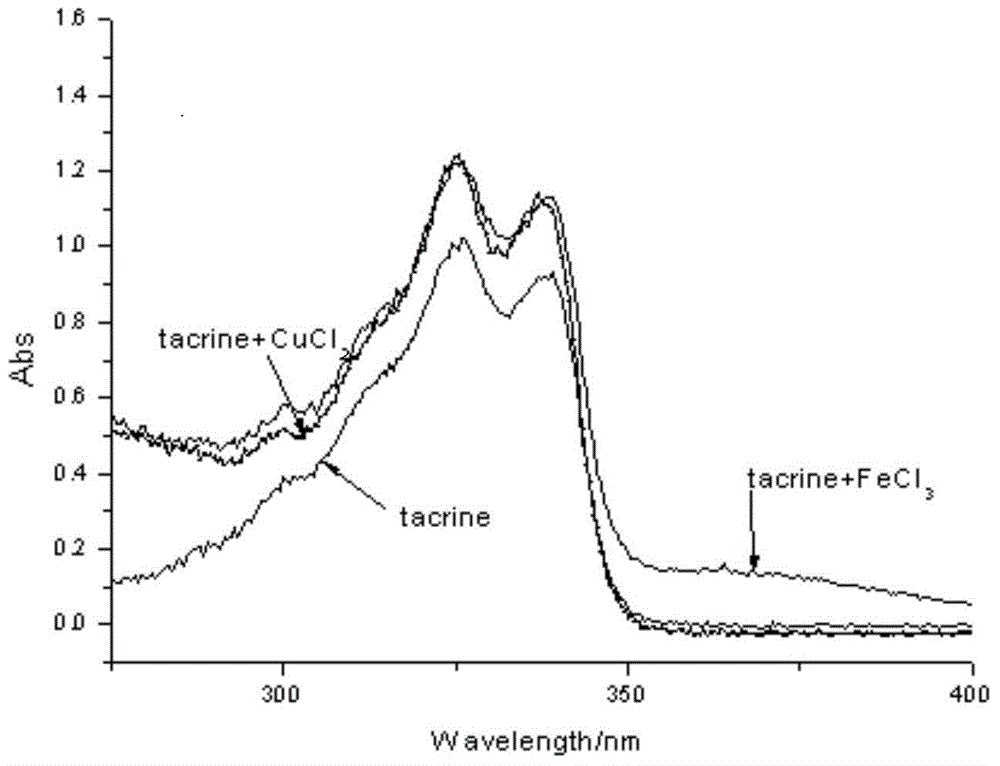
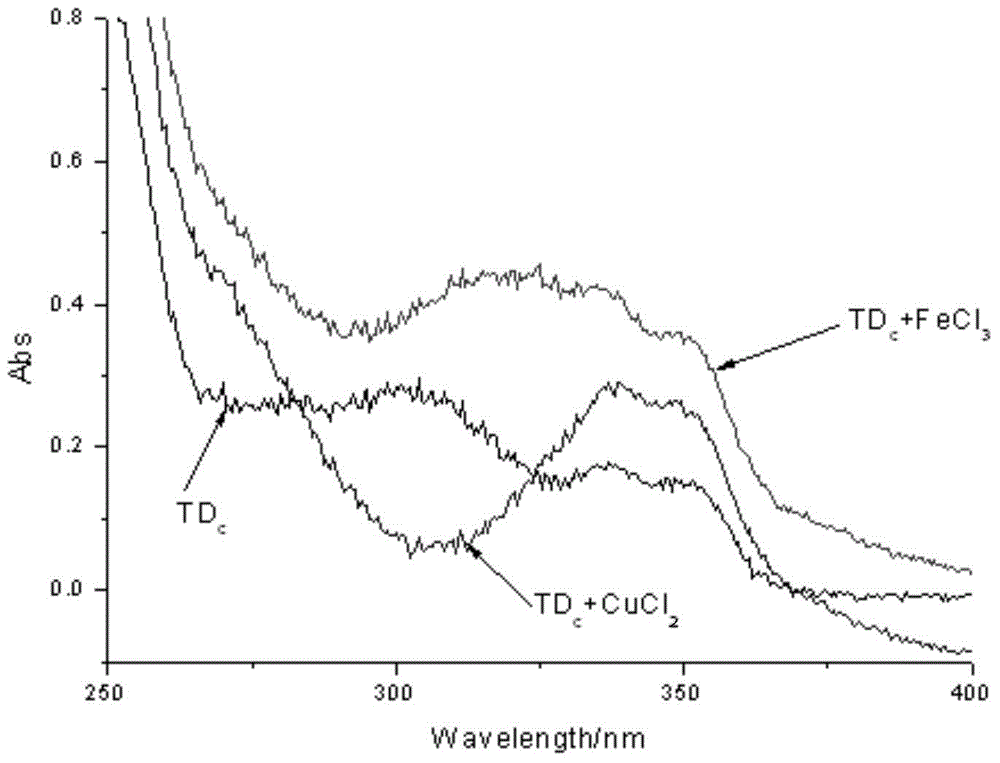
Deferasirox-tacrine metal ion chelating agent and pharmaceutical use thereof
A metal ion, tacrine technology, applied in the field of medicine and chemical industry, can solve the problems of metal ion loss and redistribution, danger, enzyme activity inhibition, etc., and achieve the effect of improving utilization efficiency and therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0037] Example 1-1 Preparation of 9-chloro-1,2,3,4-tetrahydroacridine (compound 3)
[0038] Anthranilic acid (3.2g, 23mmol) and cyclohexanone (2.26mL, 23mmol) were placed in an ice bath, and 69mmol of POCl was slowly added dropwise with a constant pressure dropping funnel 3 into the above mixture, stirring as you add. After the dropwise addition, the ice bath was removed, the above solution was heated to reflux, reacted for 3h, then cooled to room temperature, and unreacted POCl was distilled off under reduced pressure. 3 , the residue was diluted with ethyl acetate, neutralized with anhydrous potassium carbonate, washed with brine (3*100 mL). The organic layer was dried over anhydrous sodium sulfate, evaporated to dryness, and column chromatography gave 4.6 g of light yellow solid 3 with a yield of 91%. Mp 67–69°C.
Embodiment 1-2
[0039] Example 1-2 Preparation of 9-chloro-1,2,3,4-tetrahydroacridine (compound 3)
[0040] Anthranilic acid (3.2g, 23mmol) and cyclohexanone (2.26mL, 23mmol) were placed in an ice bath, and 92mmol of POCl was slowly added dropwise with a constant pressure dropping funnel 3 into the above mixture, stirring as you add. After the dropwise addition, the ice bath was removed, the above solution was heated to reflux, reacted for 2h, then cooled to room temperature, and unreacted POCl was distilled off under reduced pressure. 3 , the residue was diluted with ethyl acetate, neutralized with anhydrous potassium carbonate, washed with brine (3*100 mL). The organic layer was dried over anhydrous sodium sulfate, evaporated to dryness, and column chromatography gave 3 as a pale yellow solid. Mp 67–69°C.
Embodiment 1-3
[0041] Example 1-3 Preparation of 9-chloro-1,2,3,4-tetrahydroacridine (compound 3)
[0042] Anthranilic acid (3.2g, 23mmol) and cyclohexanone (2.26mL, 23mmol) were placed in an ice bath, and 115mmol of POCl was slowly added dropwise with a constant pressure dropping funnel 3 into the above mixture, stirring as you add. After the dropwise addition, the ice bath was removed, the above solution was heated to reflux, reacted for 4h, then cooled to room temperature, and unreacted POCl was distilled off under reduced pressure. 3 , the residue was diluted with ethyl acetate, neutralized with anhydrous potassium carbonate, washed with brine (3*100 mL). The organic layer was dried over anhydrous sodium sulfate, evaporated to dryness, and column chromatography gave a light yellow solid. Mp 67–69°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com