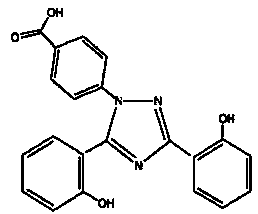
Deferasirox granules and preparation method thereof
A technology of deferasirox and granules, applied in the field of pharmaceutical preparations, can solve the problems of time-consuming and inconvenient use, limited widespread use, poor compliance and the like, and achieves the effects of convenient administration, easy dose division, and fast absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 (prescription quantity is 1000 bags)
[0029] Raw material dosage (g)
[0030] Deerasirox 125
[0031] Lactose 590
[0032] Microcrystalline Cellulose 590
[0033] 10% PVP K30 aqueous solution 75
[0034] Crospovidone 75
[0035] Sodium Lauryl Sulfate 3
[0036] Aspartame 42
[0037] Preparation process: Weigh the prescribed amount of deferasirox, lactose, microcrystalline cellulose, crospovidone, sodium lauryl sulfate and aspartame, pass through a 80-100 mesh sieve and mix thoroughly. Add an appropriate amount of 10% PVP K30 aqueous solution and stir well to make a soft material. Extrude the soft material through a 24-mesh sieve to make wet granules, and dry at 60°C for 2 hours. The dried granules are passed through a 18-mesh sieve for granulation, quality inspection, and packaging (1.5g / bag) after passing the test.
Embodiment 2
[0038] Embodiment 2 (prescription quantity is 1000 bags)
[0039] Raw material dosage (g)
[0040] Deerasirox 125
[0041] Lactose 787
[0042] Microcrystalline cellulose 393
[0043] 10% PVP K30 aqueous solution 75
[0044] Crospovidone 75
[0045] Sodium Lauryl Sulfate 3
[0046] Aspartame 42
[0047] Preparation process: Weigh the prescribed amount of deferasirox, lactose, microcrystalline cellulose, crospovidone, sodium lauryl sulfate and aspartame, pass through a 80-100 mesh sieve and mix thoroughly. Add an appropriate amount of 10% PVP K30 aqueous solution and stir well to make a soft material. Extrude the soft material through a 24-mesh sieve to make wet granules, and dry at 60°C for 2 hours. The dried granules are passed through a 18-mesh sieve for granulation, quality inspection, and packaging (1.5g / bag) after passing the test.
Embodiment 3
[0048] Embodiment 3 (prescription quantity is 1000 bags)
[0049] Raw material dosage (g)
[0050] Deerasirox 125
[0051] Lactose 885
[0052] Microcrystalline Cellulose 295
[0053] 10% PVP K30 aqueous solution 75
[0054] Crospovidone 75
[0055] Sodium Lauryl Sulfate 3
[0056] Aspartame 42
[0057]Preparation process: Weigh the prescribed amount of deferasirox, lactose, microcrystalline cellulose, crospovidone, sodium lauryl sulfate and aspartame, pass through a 80-100 mesh sieve and mix thoroughly. Add an appropriate amount of 10% PVP K30 aqueous solution and stir well to make a soft material. Extrude the soft material through a 24-mesh sieve to make wet granules, and dry at 60°C for 2 hours. The dried granules are passed through a 18-mesh sieve for granulation, quality inspection, and packaging (1.5g / bag) after passing the test.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com