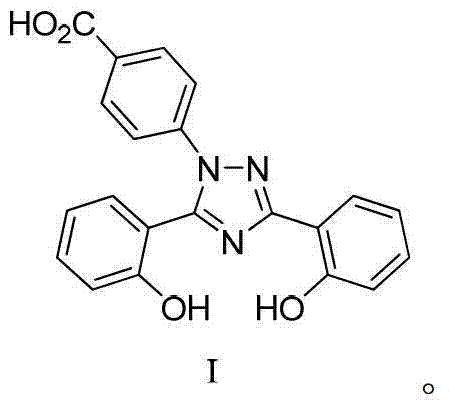
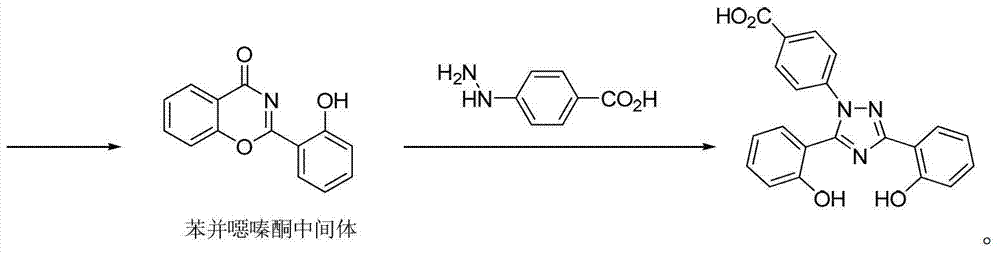
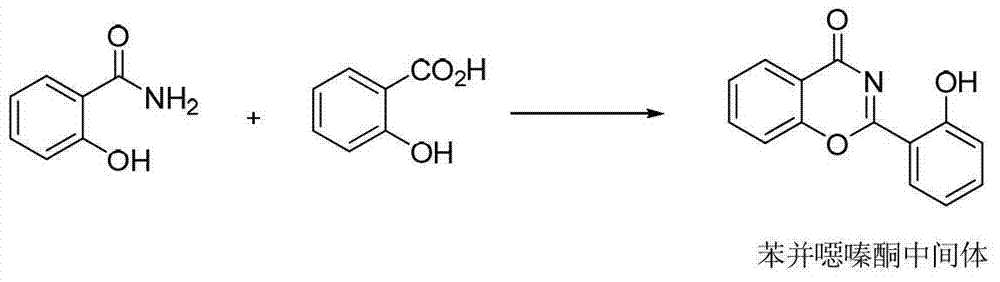
Preparation method of deferasirox and intermediate compound of deferasirox
A technology of compounds and catalysts, applied in the field of preparation methods of deferasirox and related intermediate compounds, can solve the problems of strict and cumbersome reaction process and reaction temperature control, unfavorable industrial production, etc., and achieve simple post-treatment, preparation and separation Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of o-benzyloxyphenyliminoacid methyl ester hydrochloride
[0045] Dissolve o-benzyloxybenzonitrile (31.4g, 0.15mol) in methanol (150mL), cool down to 0°C, flow HCl gas for about 2-3 hours, keep warm overnight, concentrate, add ether to the obtained solid, and obtain a white solid 39.4g, yield 90%. MS(ESI)m / z278.7(M+H) +
[0046] 1 H NMR (DMSO-d 6 ,500MHz)δ12.8(br,1H),12.2(br,1H),7.41-7.22(m,7H),6.98-7.03(m,2H),5.11(s,2H),4.45(s,3H) .
Embodiment 2
[0047] Embodiment 2: Preparation of o-benzyloxyphenyliminoacid ethyl ester hydrochloride
[0048] Dissolve o-benzyloxybenzonitrile (31.4g, 0.15mol) in absolute ethanol (150mL), cool down to 0°C, flow HCl gas for about 3-4 hours, keep warm overnight, concentrate, and add ether to the obtained solid to obtain White solid 41.3g, yield 94.7%. MS(ESI)m / z292.6(M+H) + .
[0049] 1 H NMR (DMSO-d 6,500MHz)δ12.8(br,1H),12.1(br,1H),7.38-7.18(m,7H),6.97-7.02(m,2H),5.13(s,2H),4.40(q,J= 7.0Hz,2H),1.27(t,J=7.0Hz,3H).
Embodiment 3
[0050] Embodiment 3: the preparation of formula IV compound
[0051] Add water (250mL) to o-benzyloxyphenylimidomethyl ester hydrochloride (30g, 0.11mmol), cool down to 10-20°C, add solid sodium bicarbonate (11.0g, 0.13mmol) in batches, and add the resulting mixture Dichloromethane (200 mL×3) was extracted, and the combined organic layers were dried by adding anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 25 g of o-benzyloxyphenylimidomethyl ester with a yield of 96%. MS(ESI)m / z242.5(M+H) +
[0052] 1 H NMR (DMSO-d 6 ,500MHz)δ8.78(br,1H),7.36-7.20(m,7H),6.95-7.00(m,2H),5.06(s,2H),4.41(s,3H).
[0053] Ethyl o-benzyloxyphenyliminoate can be prepared by a similar method, MS (ESI) m / z256.6 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com