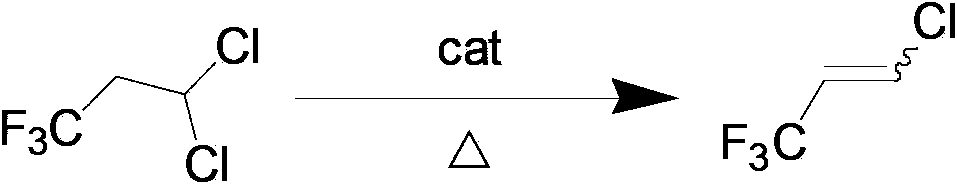
Preparation method of 1-chloro-3,3,3-trifluoropropene
A technology of trifluoropropene and trifluoropropane, applied in the field of preparation of 1-chloro-3,3,3-trifluoropropene, which can solve the problems of many by-products, low yield and low selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
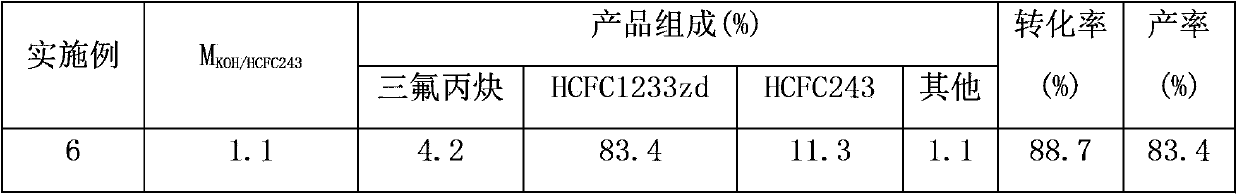
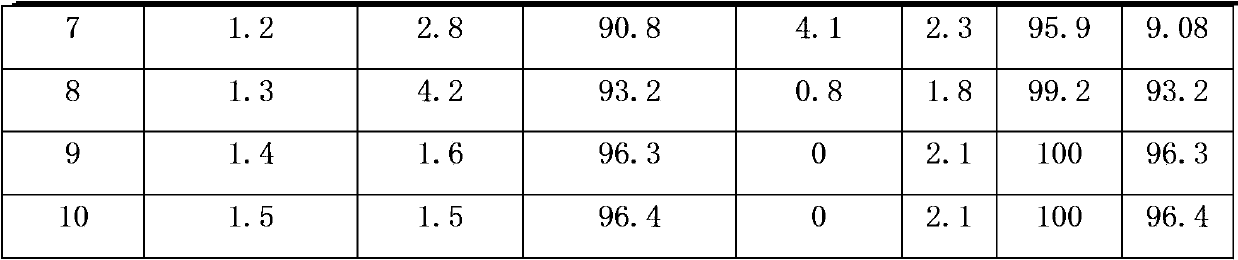
[0026] In a pressure reactor with a stirrer with a volume of 0.5L, add 1.0mol of the raw material 1,1-dichloro-3,3,3-trifluoropropane (HCFC243fa) with a mass concentration of 30% KOH solution (KOH The molar ratio with 1,1-dichloro-3,3,3-trifluoropropane is 1:1), the phase transfer catalyst tetrabutylammonium bromide is 0.01mol, and then the reactor is closed for reaction, stirring is started, The stirring speed is 200 rev / min, turn on the heating to increase the reaction temperature, the temperature is controlled at 70°C-80°C, and the reaction is carried out at constant temperature for 3.5 hours, keeping the pressure of the reaction system at 0.2MPa-0.5MPa to obtain a crude product, which is analyzed by gas chromatography. The conversion rate of HCFC243fa and the yield of HCFC1233zd are shown in Table 1 below.
Embodiment 2
[0028] The same operation as in Example 1, except that the mass concentration of KOH was changed to 40%, and the reaction results are shown in Table 1.
Embodiment 3
[0030] The same operation as in Example 1, except that the mass concentration of KOH was changed to 25%, and the reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com